Chemistry > Lab Report > Wilfrid Laurier University CH Fundamentals of Chemistry II_Experiment 6: Electrochemistry: Voltaic C (All)
Wilfrid Laurier University CH Fundamentals of Chemistry II_Experiment 6: Electrochemistry: Voltaic Cells, Electrolysis and Faraday’s Law
Document Content and Description Below
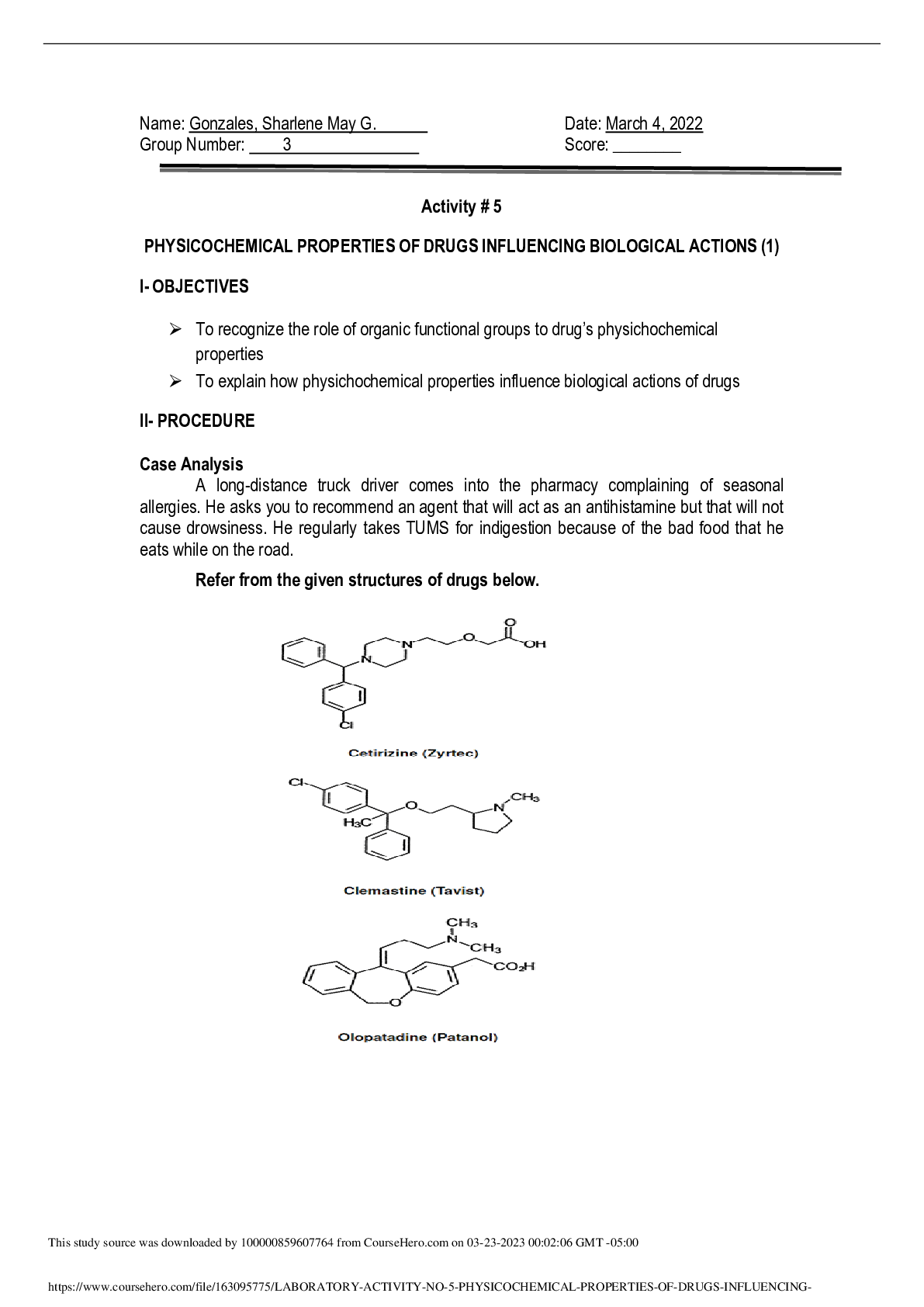
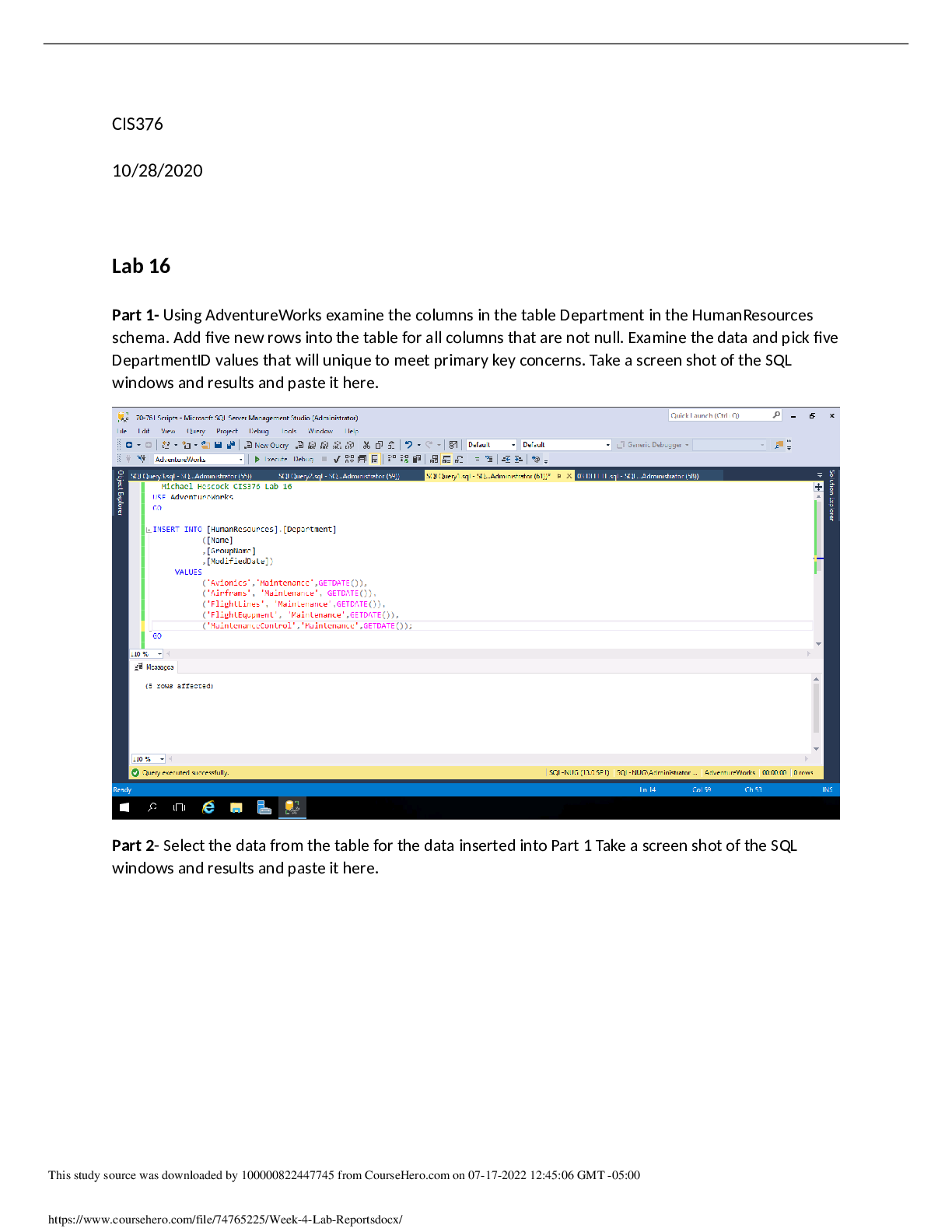
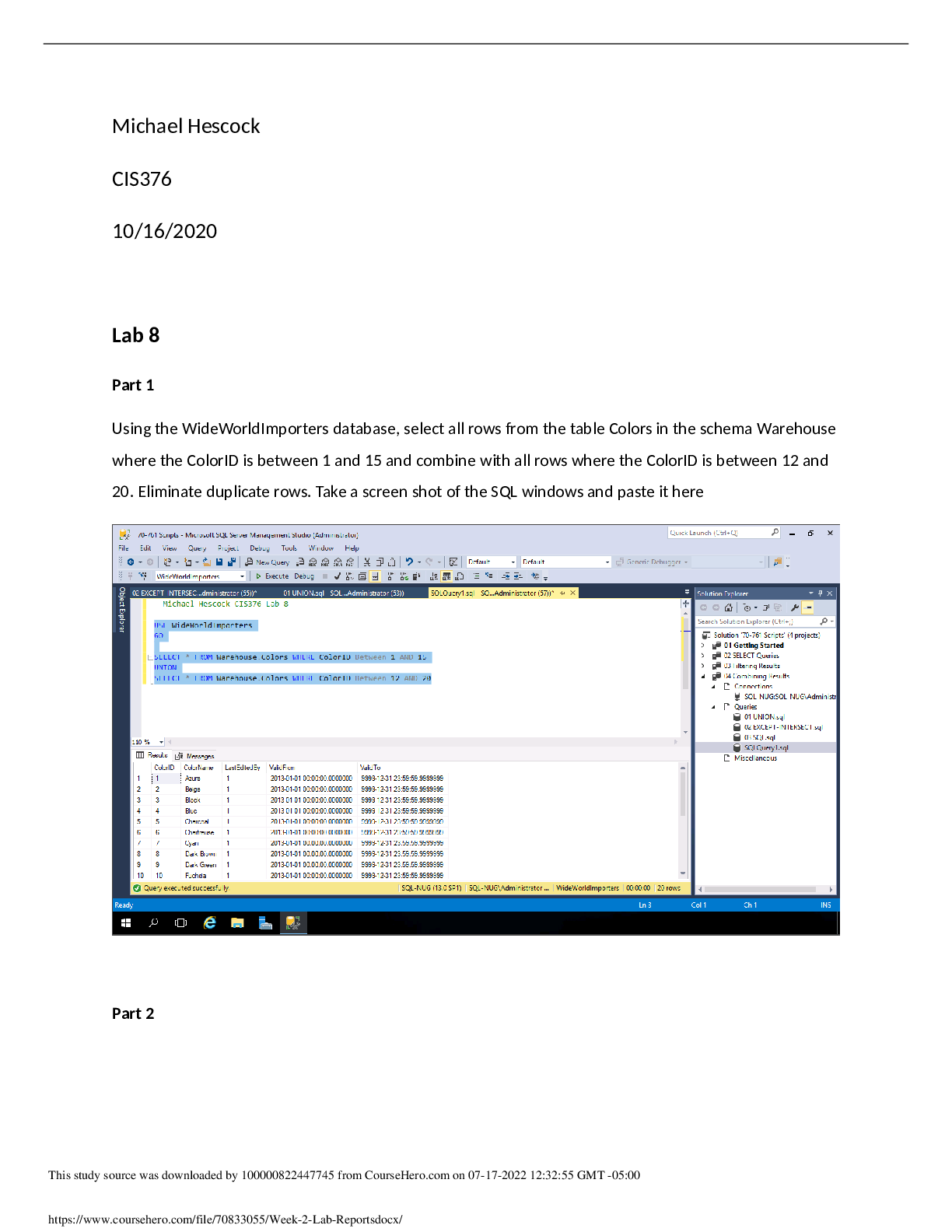
Experiment 6: Electrochemistry: Voltaic Cells, Electrolysis and Faraday’s Law Name: Sarah Bradburn Student #: 160749140 Date Conducted: March 20th, 2017 Date Submitted: March 27th, 2017 Partner... ’s Name: Sharif Kamal Lab Section: Monday 7:00, Section 3 IA’s Name: Sidney Nabuurs Abstract The purpose of this lab was to find the experimental equivalent weight of zinc and copper and compare it to their theoretical equivalent weights. This was done by the construction of a Voltaic Cell that conducted two electrolysis processes one with two copper electrodes and the other with two zinc electrodes. The amount of total charge in micro amps was obtained from an ammeter over an hour period of time. The electrodes were weighted on an analytical balance before and after the electrolysis processes. The two copper electrodes had a difference weight of 0.0305 g and -0.0298 g. The two zinc electrodes had a difference weight of -0.0335 g and 0.0301 g. The total charge transferred over the system was 91.5 C. The experimental equivalent weight of copper was 32.07 g. The experimental valence of the copper was 1.98 which had a 1% error compared to the theoretical valence which was 2. For zinc the experimental equivalent weight was 33.54 g. This was not so successful the percentage difference between the expected value of 2 valence and experimental value of 1.95 valence had a difference of 2.5%. At the end of this experiment it was concluded that it was successful. Procedure For the procedure, refer to lab manual (CH111 Lab Manual, Winter 2017) pages 88-90. Wilfrid Laurier University Chemistry Department. Winter 2017.Electrochemistry: Voltaic Cells, Electrolysis and Faraday’s Law. Pages 88-90 in Chemistry 111 Laboratory Manual. Wilfrid Laurier University, ON, Canada. Results Table 1: Qualitative Observations Substance Observation CuSO4 Dark blue, transparent liquid ZnSO4 Colourless, transparent liquid Cu strip Copper/ brown, shiny, solid metal Zn strip Silver, shiny, solid metal [Show More]
Last updated: 2 years ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$8.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 23, 2023
Number of pages
5
Written in
Additional information
This document has been written for:
Uploaded
Mar 23, 2023
Downloads
0
Views
146