Chemistry > QUESTIONS & ANSWERS > Columbia University - CHEM S35436.3.2 Test ( all answers correct ) (All)
Columbia University - CHEM S35436.3.2 Test ( all answers correct )
Document Content and Description Below
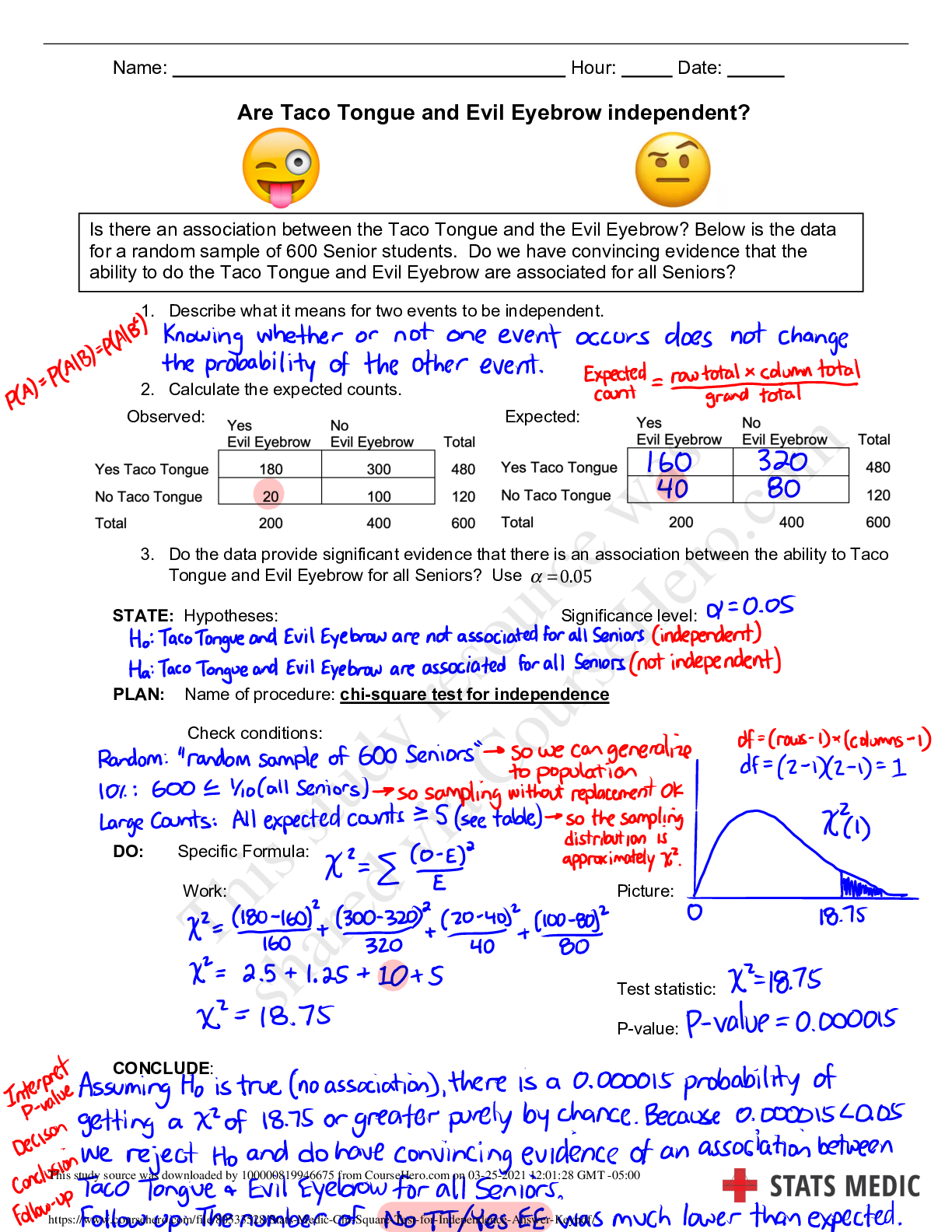
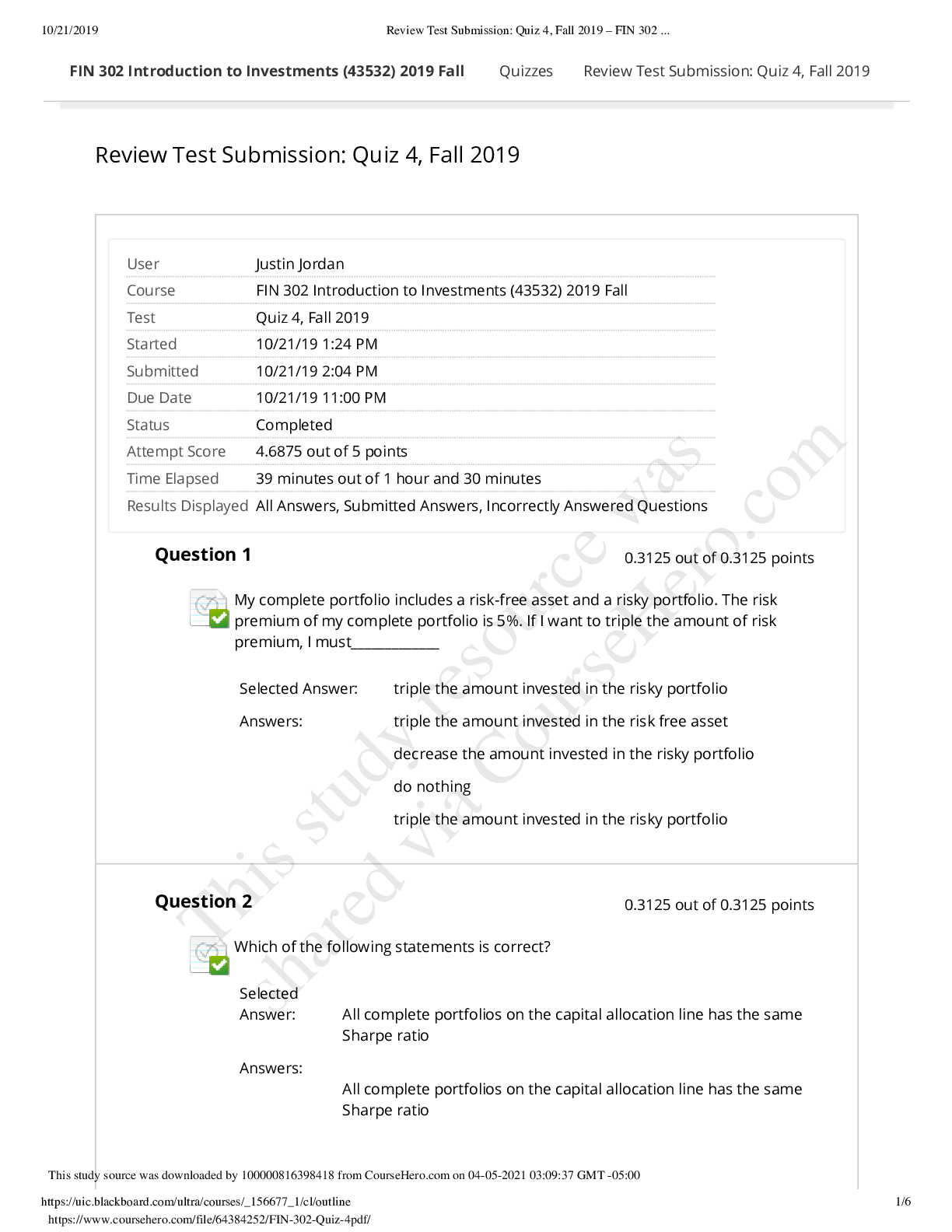
6.3.2 Test (TST): Intermolecular Forces Wrap-Up Xing Chan Question 1: Behavior of Gases (12 points) a) Write 4 – 6 sentences to describe the properties that allow a gas to be treated as an ideal ... gas. Explain and discuss the conditions in which these properties would exist. (5 points) b) The graph below describes the temperature and pressure relationship of a gas under ideal conditions. Write 2 – 3 sentences to explain the graph. Be sure to explain what will happen to the real gas behavior as the temperature of the gas is lowered. (4 points) c) Write 3 – 5 sentences to describe what happens to a can of carbonated soda when it is left in a hot car. Be sure to explain the relationship between the number of molecules, temperature, pressure, and volume. (3 points) Question 2: Solubility (10 points) a) Write 4 – 5 sentences to describe whether each of the molecules (A – C) in the image above will dissolve in water. Be sure to fully explain your reasoning, including intermolecular forces and ability to interact with water. (6 points) b) Write 4 – 5 sentences to describe how the solubility would change if molecules A – C were dissolved in benzene (C6H6). (4 points) Question 3: Chromatography (10 points) Use the following structures of amino acids to answer the questions below. Note that the difference in the structures (the side chains) is highlighted by gray shading. a) A student performed chromatography of the four amino acids and the results were shown in the chromatogram below. If an anion exchange column (column is positively charged) was used in a neutral buffer, assign each amino acid to the corresponding peak in the chromatogram. (4 points) b) Write 2 – 3 sentences to describe the interactions that will occur for each amino acid with the anion exchange column and its effect on column retention. (3 points) c) Write 2 – 3 sentences to describe how the results would differ if a cation exchange column (negatively charged column) were used. (3 points) Question 4: Bond Energy (8 points) a) Given the following table with bond lengths, explain the bonds in terms of bond energy and the relationship between locations of atoms on the periodic table. (2 points) c) Write 2 – 3 sentences to describe the bond length and bond energy of carbon-carbon bonds in single, double, and triple bonds. (2 points) Question 5: Ideal Gases (10 points) Use the image below to answer the following questions about the ideal gas law. a) Container A has 0.30 mol of H2 gas at a temperature of 85°C in a volume of 2.0 L. What is the pressure, in atm, exerted by the H2 gas molecules? (3 points) b) Container B has 2.0 g of He gas at a temperature of 85°C in a volume of 2.0 L. What is the pressure, in atm, exerted by the He gas molecules? (3 points) c) What is the total pressure, in atm, of container C if the contents of container A and container B were combined into container C, which is 2.0 L? (2 points) d) What is the total pressure, in atm, of container C if only half of the gas molecules in container A and all of the gas molecules in container B were combined? (2 points) [Show More]
Last updated: 2 years ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 17, 2021
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
Apr 17, 2021
Downloads
0
Views
79















.png)

.png)

.png)


