Chemistry > QUESTIONS & ANSWERS > Columbia University - CHEM S35436.3.2 Test (All)
Columbia University - CHEM S35436.3.2 Test
Document Content and Description Below
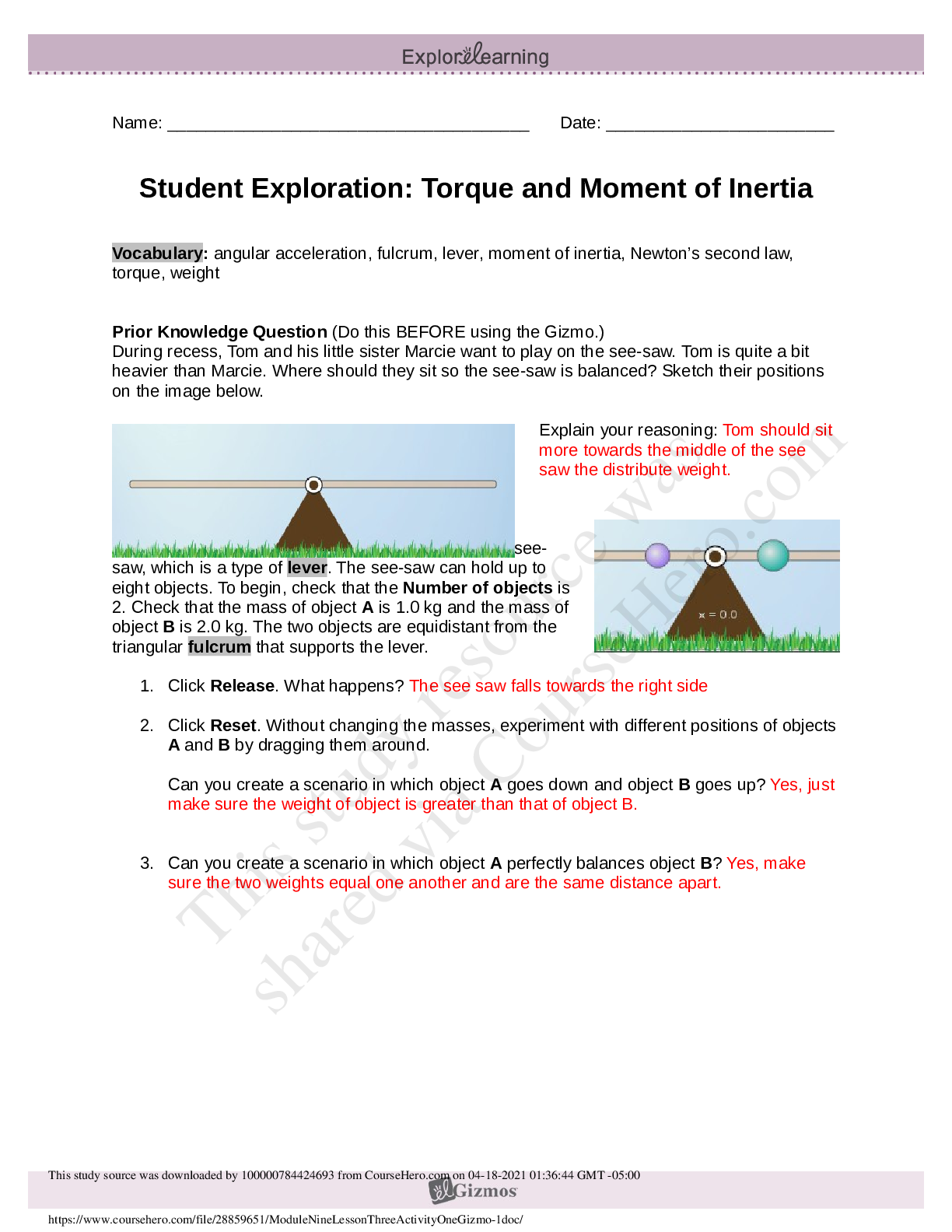
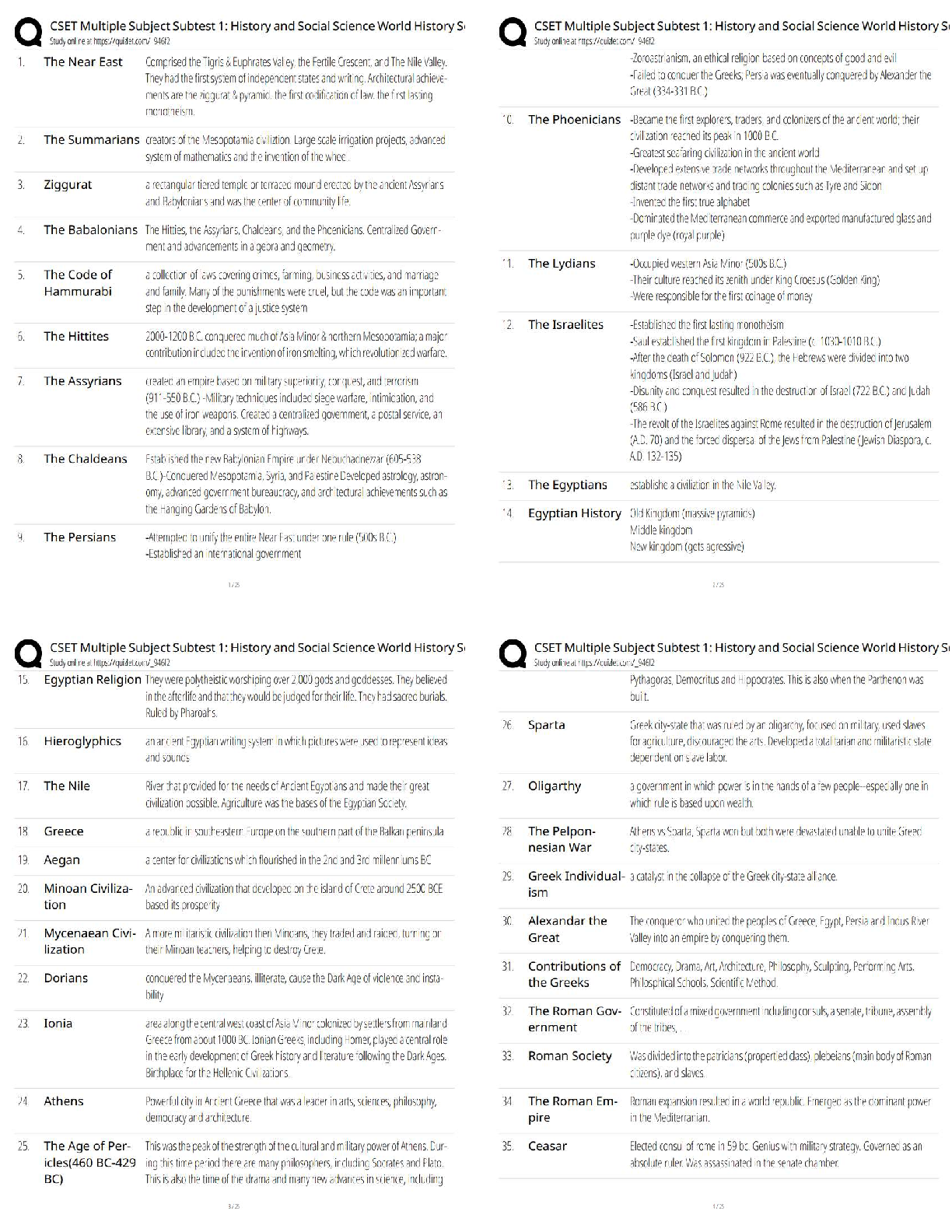
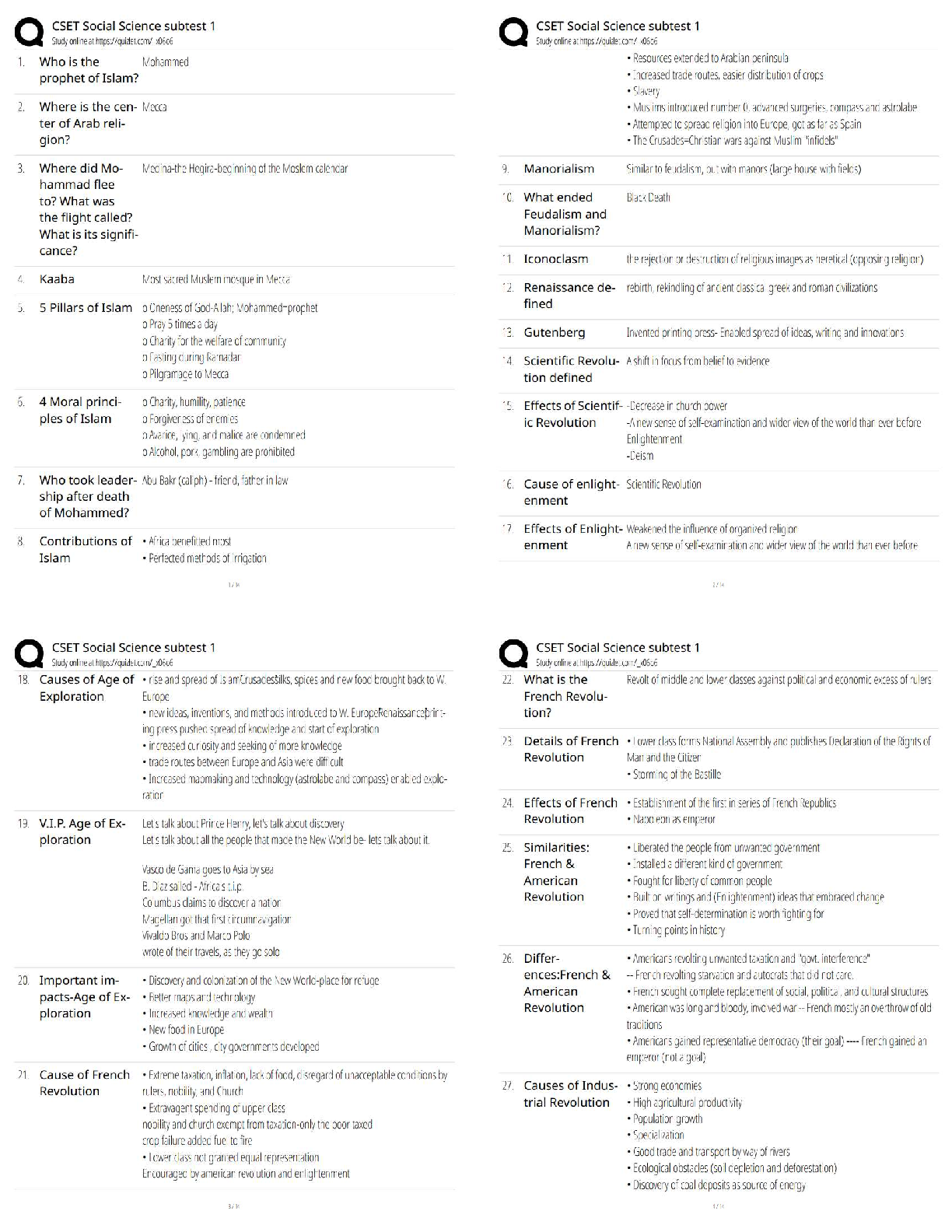
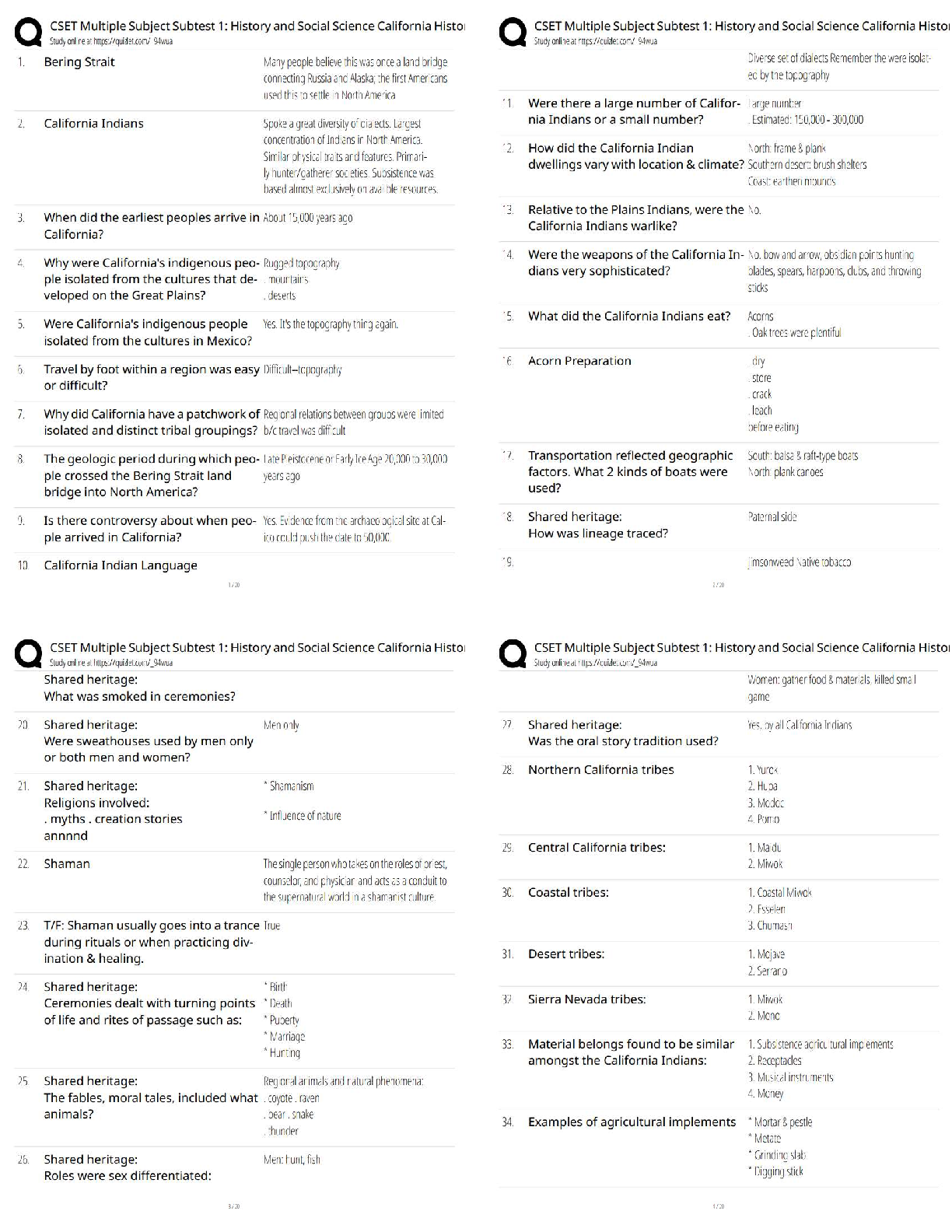
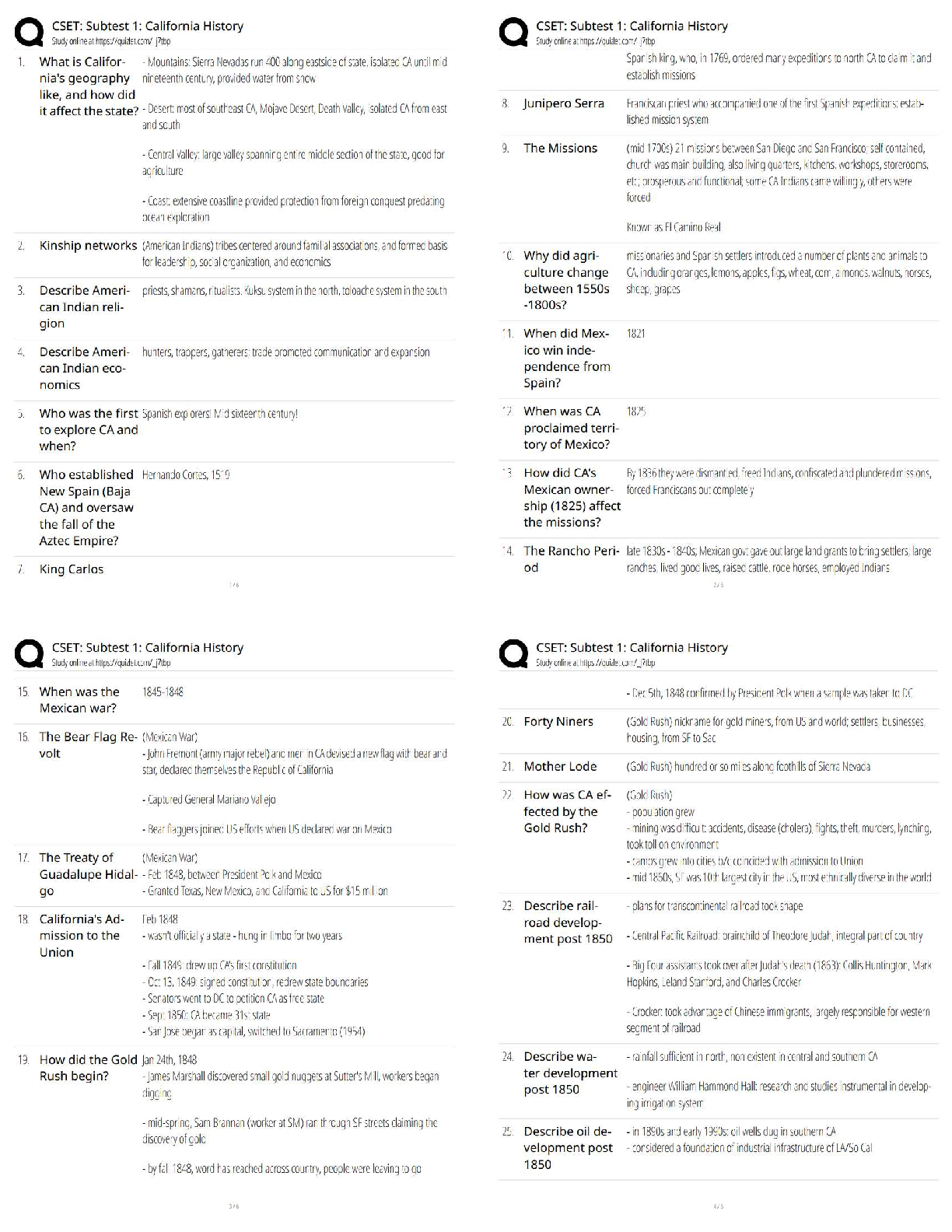
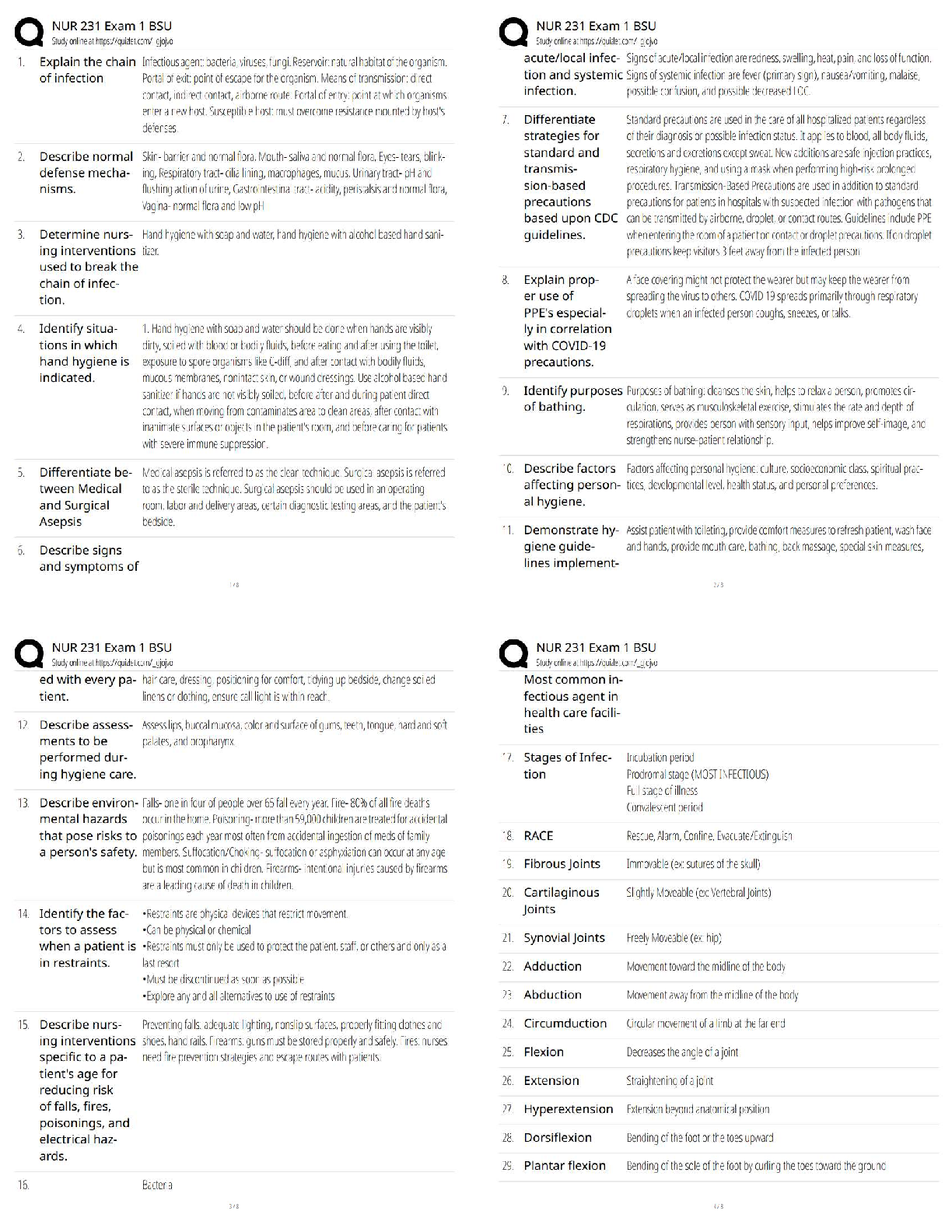
6.3.2 Test (TST): Intermolecular Forces Wrap-Up Xing Chan Question 1: Behavior of Gases (12 points) a) Write 4 – 6 sentences to describe the properties that allow a gas to be treated as an ideal ... gas. Explain and discuss the conditions in which these properties would exist. (5 points) The properties that allow a gas to be treated as an ideal gas include, the gas particles having negligible volume, the gas particles are equally sized and do not have intermolecular forces with other gas particles, the gas particles move randomly in agreement with Newton’s Laws of Motion, and finally the gas particles having perfect elastic collisions with no energy loss. But in reality, there are no ideal gases. However, gases can be considered ideal in either low pressure or high-temperature systems. b) The graph below describes the temperature and pressure relationship of a gas under ideal conditions. Write 2 – 3 sentences to explain the graph. Be sure to explain what will happen to the real gas behavior as the temperature of the gas is lowered. (4 points) As the temperature is lowered, the kinetic energy of the gas molecules decreases. Eventually, a point is reached where the molecules can no longer overcome the intermolecular attractive forces, and the gas liquefies. c) Write 3 – 5 sentences to describe what happens to a can of carbonated soda when it is left in a hot car. Be sure to explain the relationship between the number of molecules, temperature, pressure, and volume. (3 points) As the temperature inside the car increases, high heat can start to affect the taste and consistency of the carbonated soda. Heat can affect some soda ingredients, changing the flavor of the drink. In the extreme heat, the number of molecules start moving more rapidly, and the can could explode due to heat creating extreme pressure inside the [Show More]
Last updated: 3 years ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 17, 2021
Number of pages
8
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 17, 2021
Downloads
0
Views
177





.png)