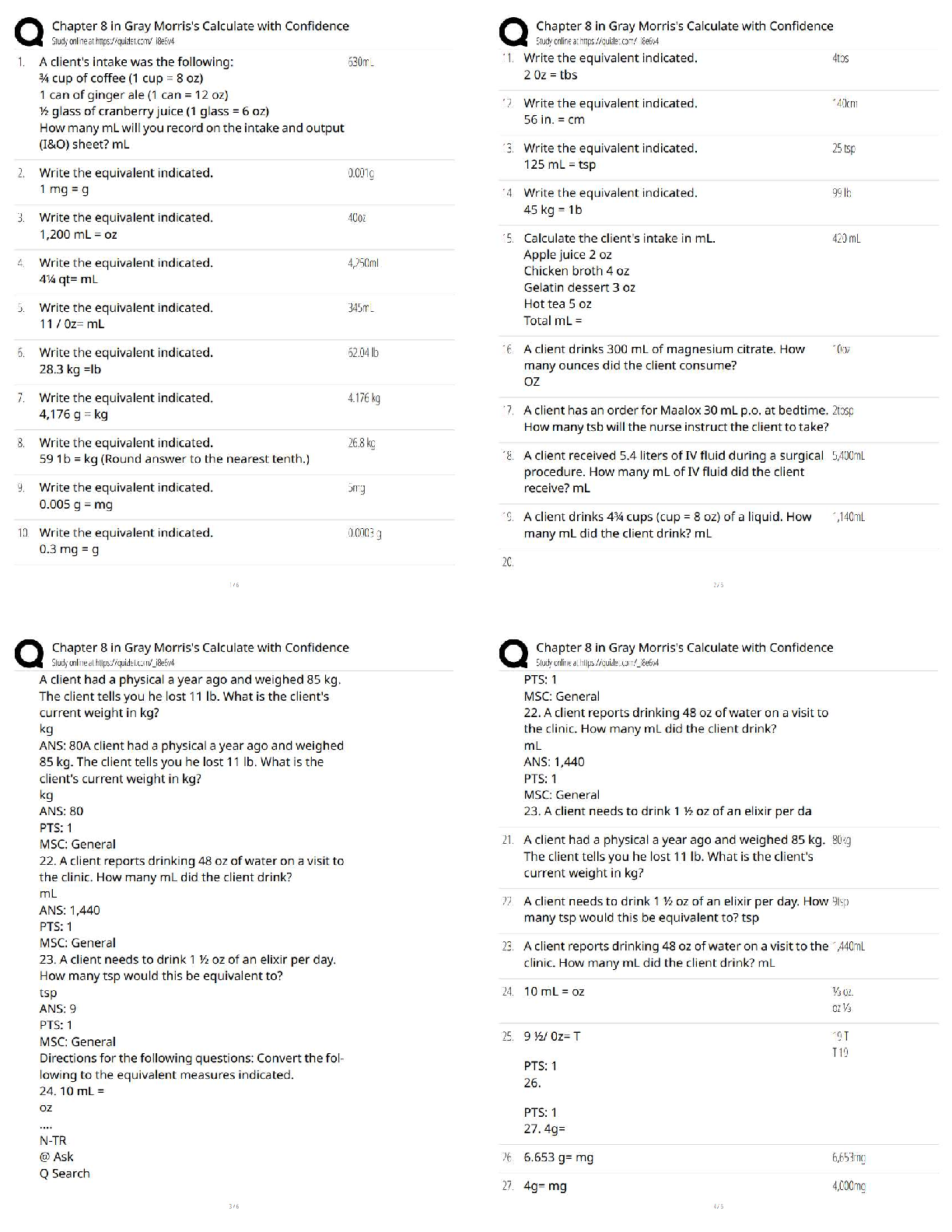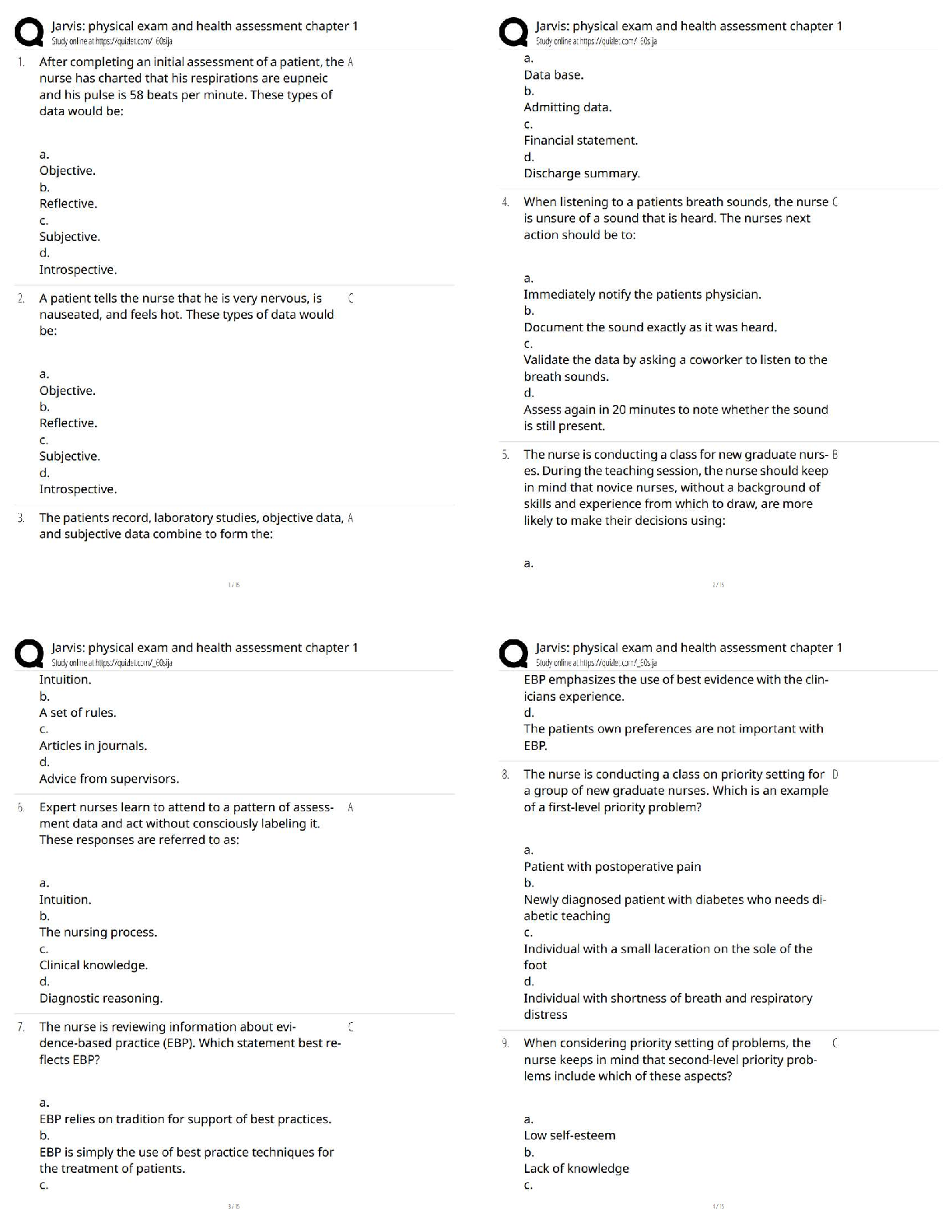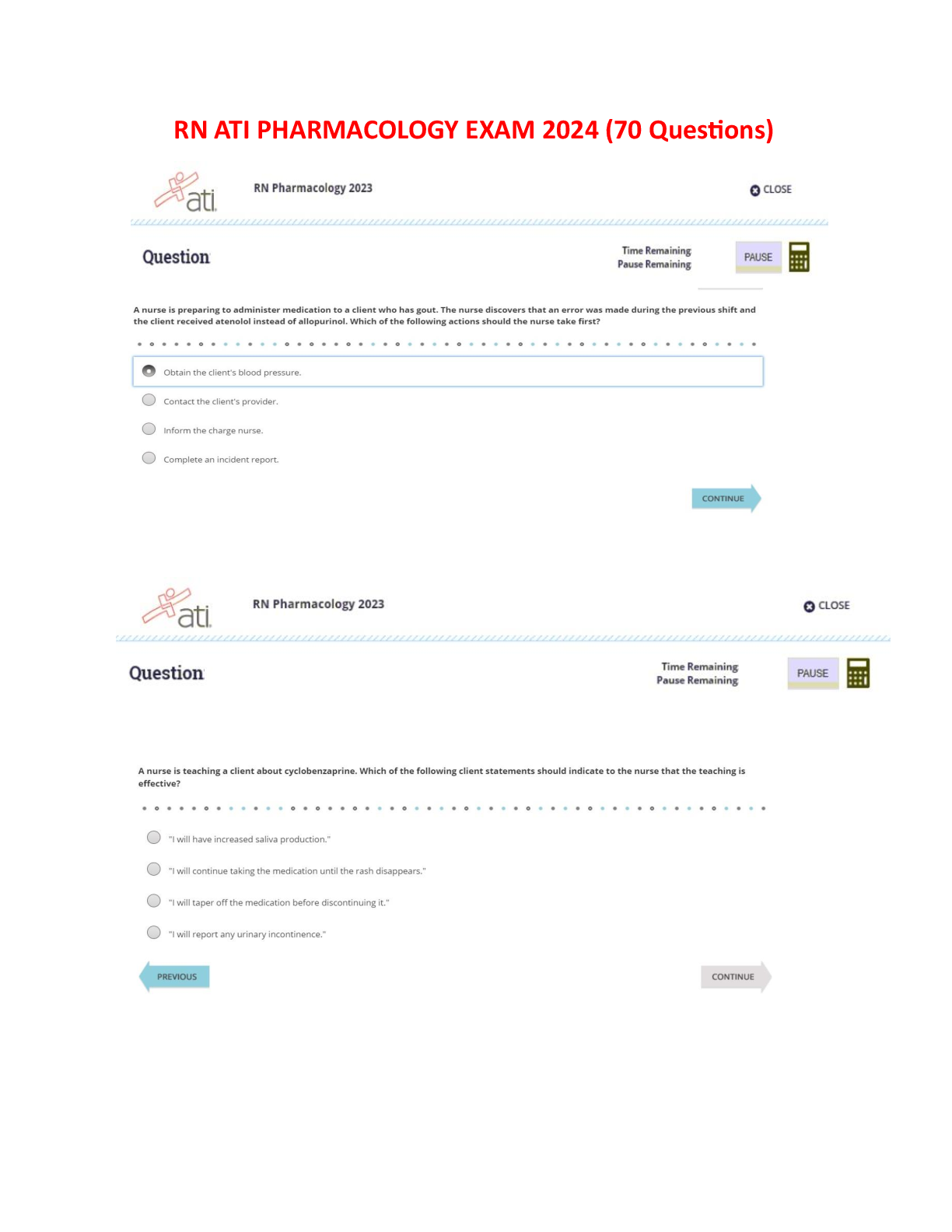
RN PHARMACOLOGY 2023 (70Questions)
$ 40
.png)
Sophia (Macroeconomics) - Unit 3 Milestone Questions and Answers Already Solved
$ 8

ATI TEAS 7 - English & Language Usage(2022