Chemistry > EXAM > Los Osos High CHEMISTRY 101; Student Exploration: Photoelectric Effect Gizmos (answered) Fall 2021 (All)
Los Osos High CHEMISTRY 101; Student Exploration: Photoelectric Effect Gizmos (answered) Fall 2021
Document Content and Description Below
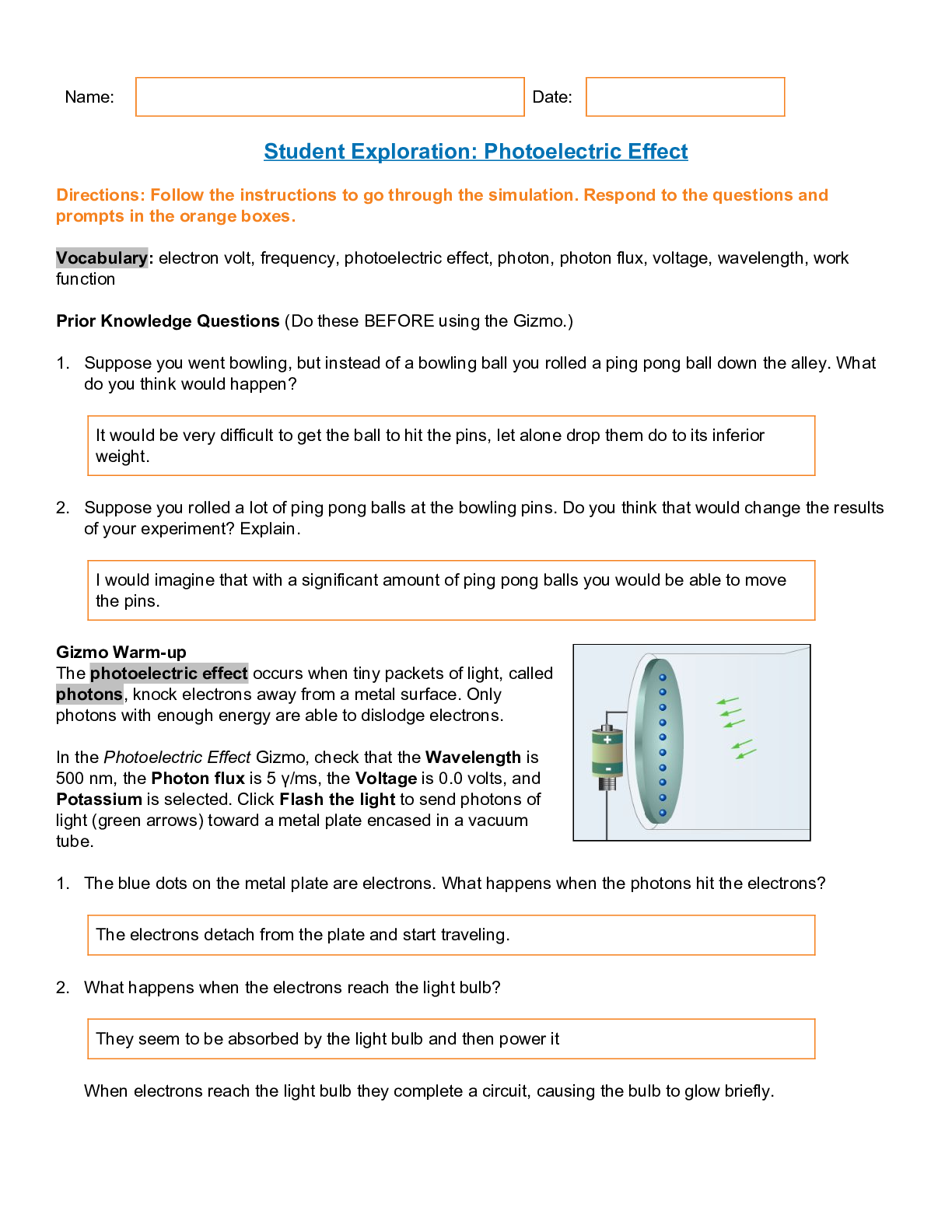
Los Osos High CHEMISTRY 101; Student Exploration: Photoelectric Effect Gizmos (answered) Fall 2021-Gizmos Student Exploration: Photoelectric Effect Prior Knowledge Questions (Do these BEFORE using ... the Gizmo.) 1. Suppose you went bowling, but instead of a bowling ball you rolled a ping pong ball down the alley. What do you think would happen? 2. Suppose you rolled a lot of ping pong balls at the bowling pins. Do you think that would change the results of your experiment? Explain. Gizmo Warm-up The photoelectric effect occurs when tiny packets of light, called photons, knock electrons away from a metal surface. Only photons with enough energy are able to dislodge electrons. In the Photoelectric Effect Gizmo, check that the Wavelength is 500 nm, the Photon flux is 5 γ/ms, the Voltage is 0.0 volts, and Potassium is selected. Click Flash the light to send photons of light (green arrows) toward a metal plate encased in a vacuum tube. 1. The blue dots on the metal plate are electrons. What happens when the photons hit the electrons? 2. What happens when the electrons reach the light bulb? Introduction: Through the centuries, many scientists have debated whether light is a wave or a stream of tiny particles. In the 1800s, most scientists agreed that phenomena such as refraction and diffraction supported the “light as a wave” theory. However, Albert Einstein’s explanation of the photoelectric effect showed that light can act like a stream of particles as well. Question: What factors affect the ability of light to free electrons from a metal surface? 1. Observe: Click Flash the light with a variety of wavelength values. What do you notice? 2. Observe: The photon flux is a measure of how bright the light is. It is equal to the number of photons that are released in a given time. It is given as photons (γ) per millisecond (ms). Click Flash the light with a variety of Photon flux values. What do you notice? 3. Form hypothesis: Answer the following questions based on what you have observed so far. 4. Investigate: Set the Photon flux to 1 γ/ms. Use the Gizmo to find the longest wavelength that will dislodge an electron from the metal surface. What is this wavelength? 5. Predict: Set the Wavelength to 540 nm. What do you think will happen if you flash the light with a photon flux of 1 γ/ms? What if you flash the light with a flux of 10 γ/ms? 6. Test: Click Flash the light with a Photon flux of 1 γ/ms and again with a flux of 10 γ/ms. What happened? 7. Explore: Set the Wavelength to 400 nm. Experiment with different photon fluxes. 8. Infer: For mechanical waves, such as sound waves or ocean waves, increasing the intensity of the wave increases both the amplitude (height) of the wave and the energy it carries. In that situation, a low-frequency but high-intensity wave should have the same effect as a high-frequency but low-intensity wave. How does light behave differently from this model? 9. Think and discuss: How is firing photons at the surface of a metal analogous to rolling different types of balls at a set of bowling pins? If possible, discuss your answer with your classmates and teacher. Introduction: The electrons that are freed from the surface of the metal have a specific amount of kinetic energy. Faster electrons have greater energies than slower ones. The energy of emitted electrons is measured by setting up an electrical field that opposes their motion. The voltage of the field is a measure of its strength. Goal: Use a voltage gradient to measure the energy of emitted electrons. 1. Observe: Check that Potassium is selected. Click Flash the light and observe the emitted electrons. Increase the Voltage to 1.5 volts, and click Flash the light again. How does the electrical field affect the motion of the emitted electrons? 2. Measure: The energy of an emitted electron is measured in electron volts (eV). An electron with an energy of 1 eV can overcome an electrical field of 1 volt. In the Gizmo, increase the voltage until you find the highest voltage that still allows the electrons to reach the light bulb. 3. Gather data: With the Wavelength set to 300 nm, measure the energy of emitted electrons for potassium, calcium, and uranium. Then measure the same values with wavelengths of 250 nm and 200 nm to complete the table. 4. Analyze: What patterns do you notice in your data? 5. Infer: Based on your data, which element is hardest to extract electrons from? Introduction: It is easier to remove electrons from some elements than others. The energy required to free an electron from the surface of a solid is the work function of the element. Question: How much energy is required to liberate electrons from a material? 1. Predict: In general, the difficulty of removing electrons increases from left to right across each row of the periodic table. Look up potassium (K), calcium (Ca), and uranium (U). Based on their positions in the periodic table, which of these elements do you expect to have the lowest work function? Which element will have the highest work function? 2. Gather data: Use the Gizmo to determine the highest wavelength for each element that still removes electrons. Fill in the first column below. [Show More]
Last updated: 2 years ago
Preview 1 out of 6 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 16, 2021
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Nov 16, 2021
Downloads
0
Views
98
















