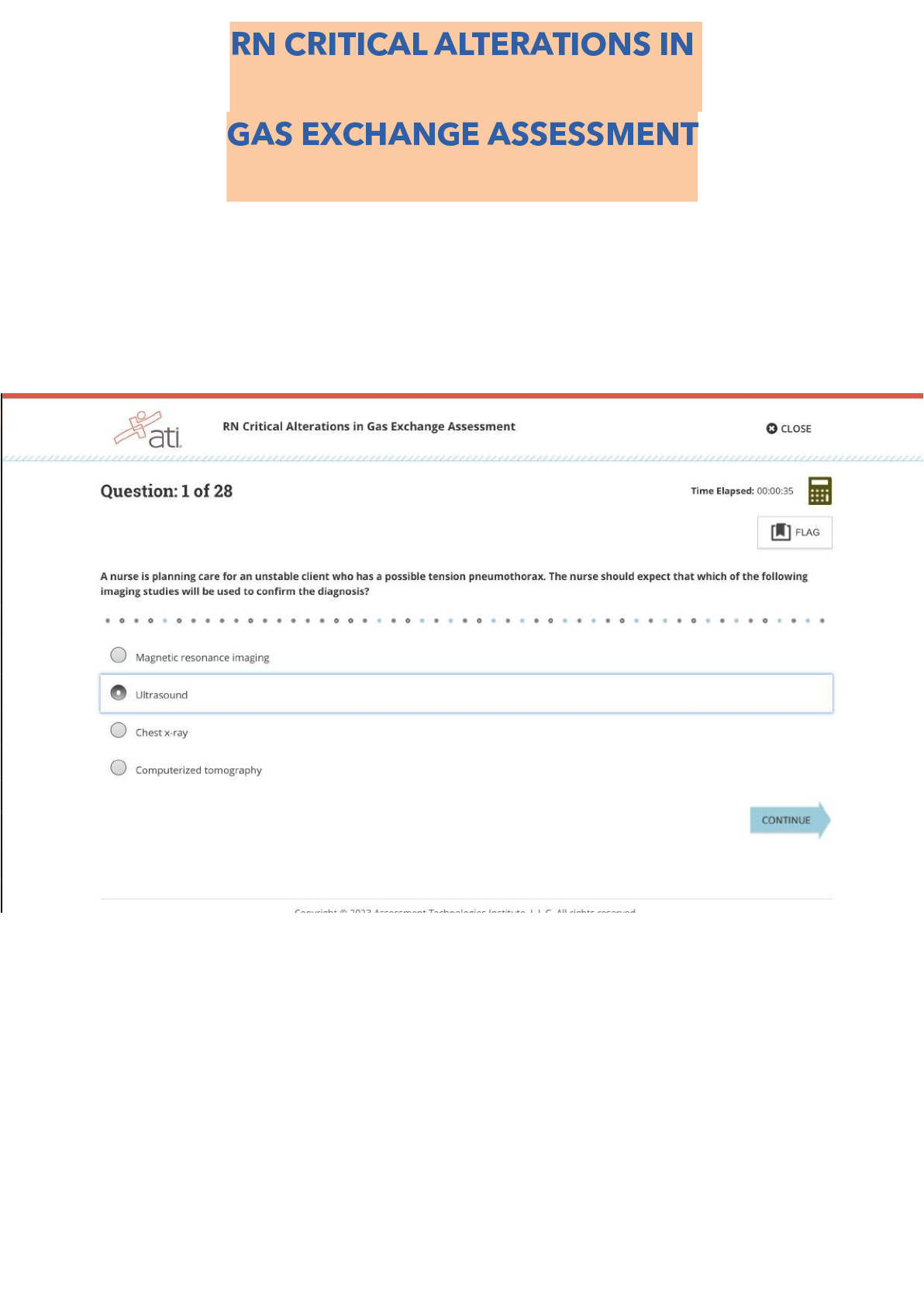
ATI RN Critical Alterations in Gas Exchange Assessment
Chemistry > EXAM REVIEW > AQA CHEMISTRY 2 COMPLETE SOLUTION GUIDE (All)
Last updated: 3 years ago
Preview 1 out of 32 pages
Instant download

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Can't find what you want? Try our AI powered Search
Connected school, study & course
About the document
Uploaded On
Dec 01, 2021
Number of pages
32
Written in
All
This document has been written for:
Uploaded
Dec 01, 2021
Downloads
0
Views
152
Scholarfriends.com Online Platform by Browsegrades Inc. 651N South Broad St, Middletown DE. United States.
We're available through e-mail, Twitter, and live chat.
FAQ
Questions? Leave a message!
Copyright © Scholarfriends · High quality services·