Chemistry > QUESTIONS & ANSWERS > CHEM120 Week 1 to Week 7 Quizzes / CHEM 120 Week 1 to Week 7 Quizzes (GRADED A):Chamberlain College (All)
CHEM120 Week 1 to Week 7 Quizzes / CHEM 120 Week 1 to Week 7 Quizzes (GRADED A):Chamberlain College of Nursing |100% CORRECT
Document Content and Description Below
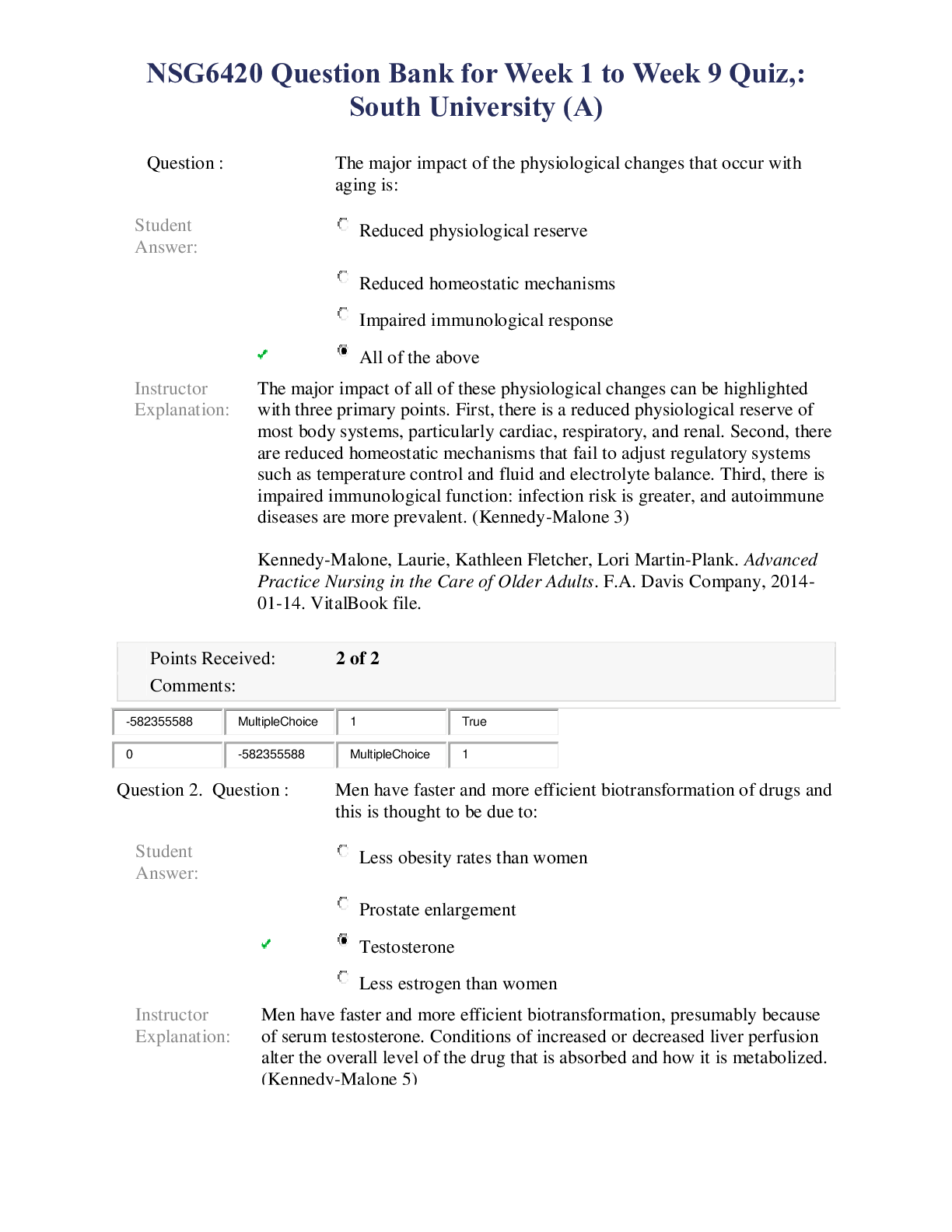
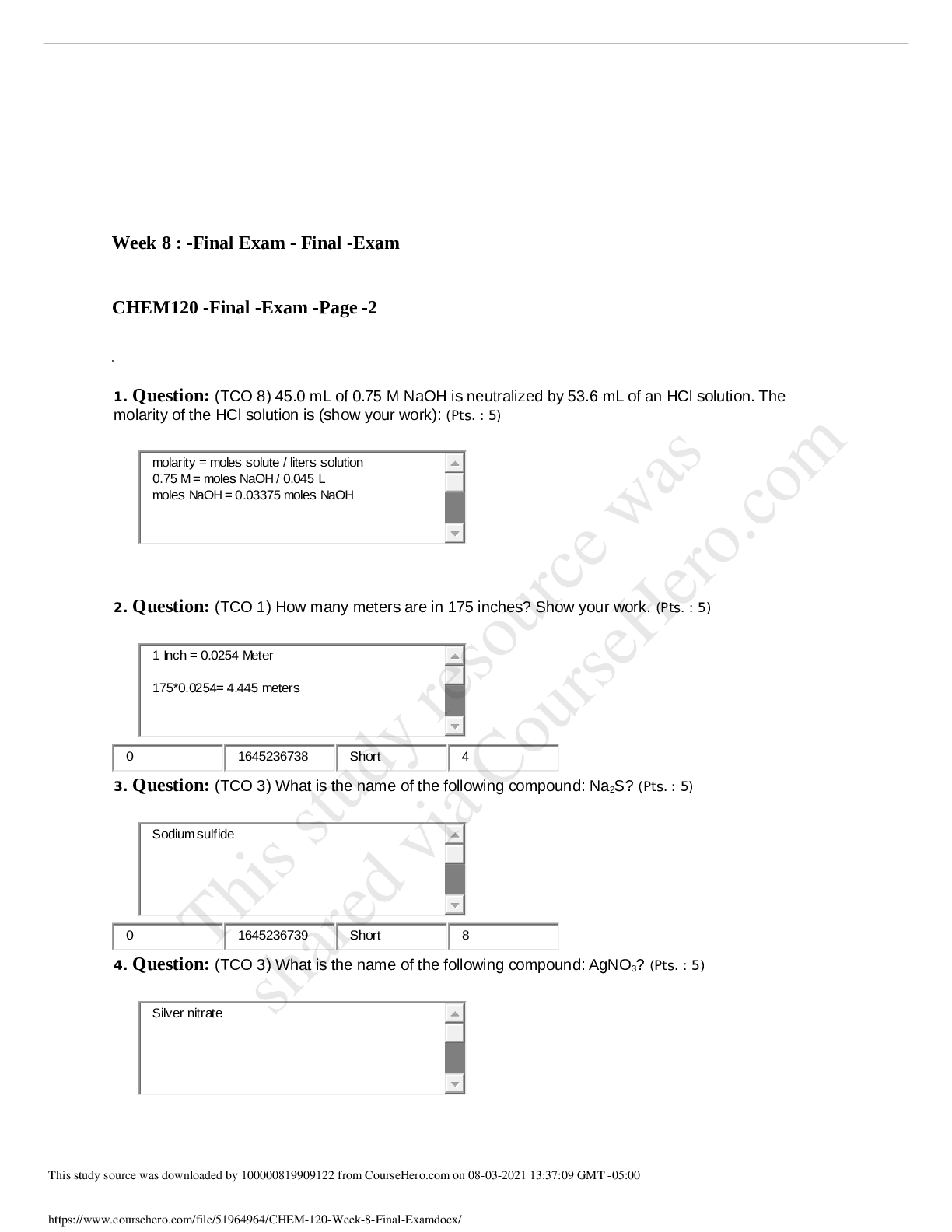
1. Question : (TCO 2) The atomic number of an atom is equal to the number of Student Answer: electrons plus protons. nuclei. neutrons plus protons. protons. neutrons. Instructor Explanation: ... Chapter 3; The atomic number = number of protons 1921547766 MultipleChoice 2 True 0 1921547766 MultipleChoice 2 2. Question : (TCO 2) Consider a neutral atom with 30 protons and 34 neutrons. The atomic number of the element is Student Answer: 94. 30. 64. 32. 34. Instructor Explanation: Chapter 3; Atomic number = number of protons. Therefore, in this case, the atomic number = 30. 1921547767 MultipleChoice 5 0 1921547767 MultipleChoice 5 3. Question : (TCO 2) No matter how much extra oxygen is available, 12 grams of carbon always combines with 32 grams of oxygen. This best illustrates the law of Student Answer: conservation of mass. 1. Question : (TCO 3) The maximum number of electrons that may reside in the n=2 energy level is Student Answer: 2. 3. 8. 10. 18. Instructor Explanation: Chapter 3 1926461364 MultipleChoice 2 True 0 1926461364 MultipleChoice 2 2. Question : (TCO 3) How many electrons are in a phosphorus atom? Student Answer: 15 19 23 39 84 Instructor Explanation: Chapter 3 1926461365 MultipleChoice 5 True 0 1926461365 MultipleChoice 5 3. Question : (TCO 3) Which is an impossible electron configuration? Student Answer: 1s2 2s2 1s2 2s2 2p4 1s2 2s2 2p4 1. Question : (TCO 5) A reaction that releases energy as it occurs is classified as a(n) _____. Student Answer: catalyzed reaction exothermic reaction decomposition reaction endothermic reaction oxidation-reduction reaction Instructor Explanation: Week 3 Lecture; Reactions that release energy are exothermic. 1931785666 MultipleChoice 1 True 0 1931785666 MultipleChoice 1 2. Question : (TCO 5) What should be the coefficient of hydrogen, H2, in the following [Show More]
Last updated: 2 years ago
Preview 1 out of 66 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 24, 2022
Number of pages
66
Written in
Additional information
This document has been written for:
Uploaded
Jan 24, 2022
Downloads
0
Views
142




















.png)


