Chemistry GIzmo 1.docRATED A_+
Document Content and Description Below
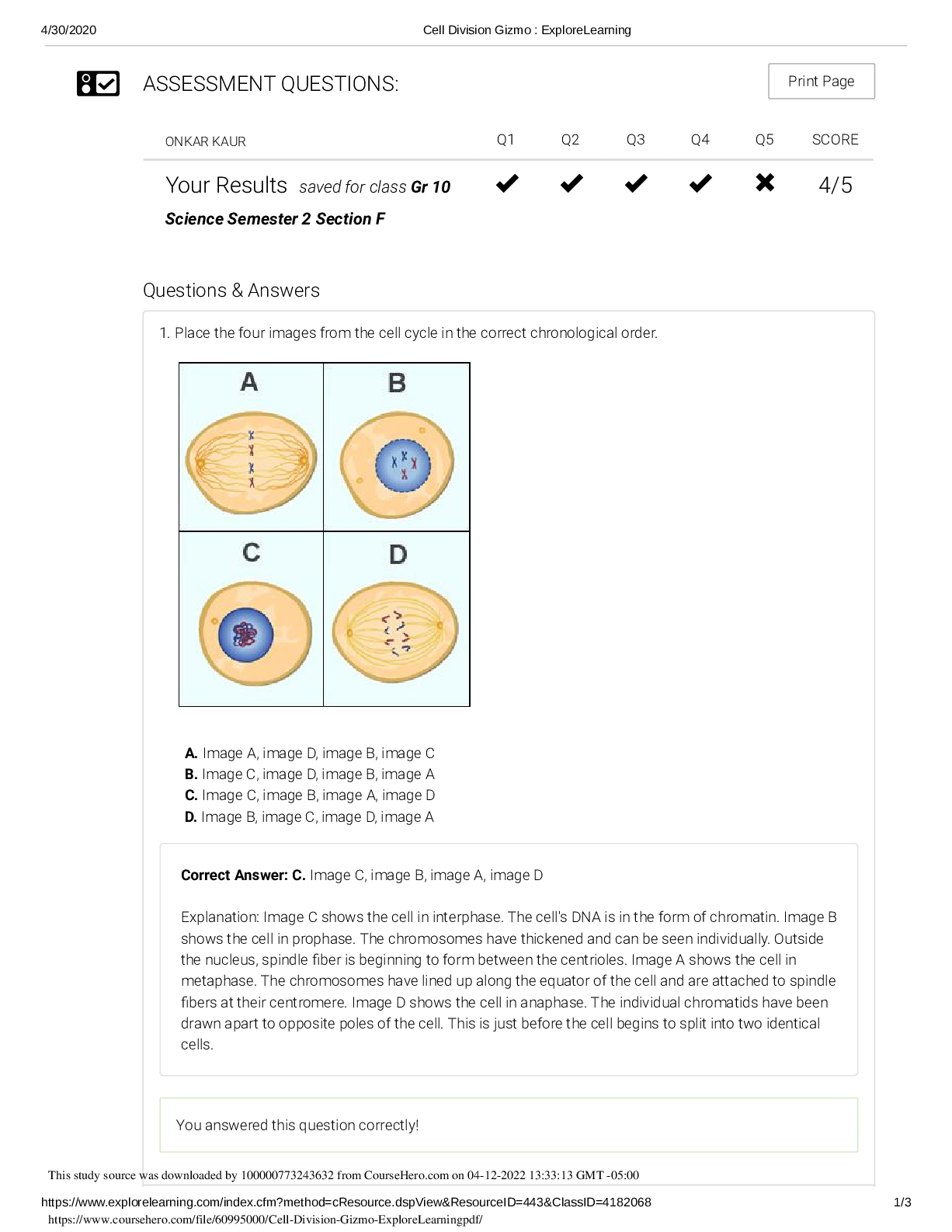
Student Exploration: Density Laboratory NCVPS Chemistry Fall 2014 Vocabulary: buoyancy, density, graduated cylinder, mass, matter, scale, volume Prior Knowledge Questions (Do these BEFORE using the ... Gizmo.) 1. Of the objects below, circle the ones you think would float in water. If you cannot circle them on your computer, simply type the names here: Cruise ship, beach ball. 2. Why do some objects float, while others sink? Since they are solids, they are heavy and cannot stay afloat. Gizmo Warm-up The Density Laboratory Gizmo™ allows you to measure a variety of objects, then drop them in water (or other liquid) to see if they sink or float. 1. An object’s mass is the amount of matter it contains. The mass of an object can be measured with a calibrated scale like the one shown in the Gizmo. Drag the first object onto the Scale. (This is object 1.) What is the mass of object 1? 19.5g 2. An object’s volume is the amount of space it takes up. The volume of an irregular object can be measured by how much water it displaces in a graduated cylinder. Place object 1 into the Graduated Cylinder. What is the volume of object 1? 14 mL Note: While milliliters (mL) are used to measure liquid volumes, the equivalent unit cubic centimeters (cm3 ) are used for solids. Therefore, write the volume of object 1 in cm3 . 3. Drag object 1 into the Beaker of Liquid. Does it sink or float? sink This study source was downloaded by 100000773243632 from CourseHero.com on 03-29-2022 04:04:46 GMT -05:00 https://www.coursehero.com/file/38132079/Chemistry-GIzmo-1doc/ Activity A: Float or sink? Get the Gizmo ready: Drag object 1 back to the shelf. Check that Liquid Density is set to 1.0 g/mL. Question: How can you predict whether an object will float or sink? 1. Observe: Experiment with the different objects in the Gizmo. Try to determine what the floating objects have in common and what the sinking objects have in common. 2. Form hypothesis: Compare the floating objects, then do the same for the sinking objects. A. What do the floating objects have in common? The majority of the objects that floated had a density of less than 1. B. What do the sinking objects have in common? Most of the sinking objects had a density much higher than the water. 3. Collect data: Measure the mass and volume of objects 1 through 12, and record whether they float or sink in the table below. Leave the last column blank for now. Object Mass (g) Volume (cm3 ) Float or sink? Density(g/cm^ 3) 1 19.5 14 Sink 1.39 2 11 9 Sink 1.22 3 4 5 Float 0.8 4 135 7 Sink 19.28 5 4 3.5 Sink 1.14 6 78 29 Sink 2.68 7 2 21 Float 0.09 8 24 26 Float 0.92 9 99 44 Sink 2.25 10 42 61 Float 0.68 11 65 40 Sink 1.625 12 104 114 Float 0.91 [Show More]
Last updated: 3 years ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 29, 2022
Number of pages
5
Written in
All
Additional information
This document has been written for:
Uploaded
Mar 29, 2022
Downloads
0
Views
116