Chemistry > GIZMOS > CHEM 124007/September 3 - September 9/Solvay Process. Graded A+. Down;load for comprehension (All)
CHEM 124007/September 3 - September 9/Solvay Process. Graded A+. Down;load for comprehension
Document Content and Description Below
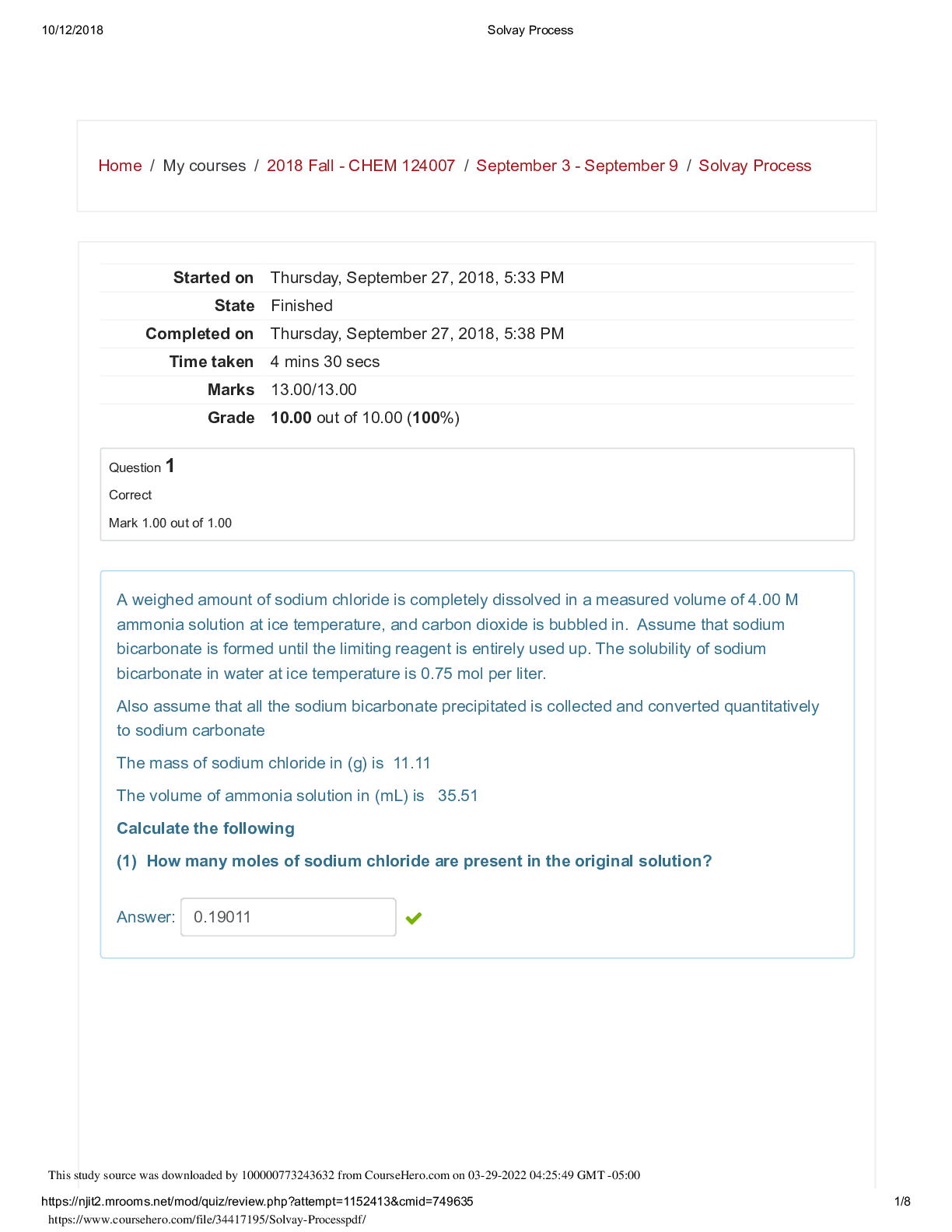
A weighed amount of sodium chloride is completely dissolved in a measured volume of 4.00 M ammonia solution at ice temperature, and carbon dioxide is bubbled in. Assume that sodium bicarbonate is fo... rmed until the limiting reagent is entirely used up. The solubility of sodium bicarbonate in water at ice temperature is 0.75 mol per liter. Also assume that all the sodium bicarbonate precipitated is collected and converted quantitatively to sodium carbonate The mass of sodium chloride in (g) is 11.11 The volume of ammonia solution in (mL) is 35.51 Calculate the following (1) How many moles of sodium chloride are present in the original solution? Answer: 0.19011 This study source was downloaded by 100000773243632 from CourseHero.com on 03-29-2022 04:25:49 GMT -05:00 https://www.coursehero.com/file/34417195/Solvay-Processpdf/ 10/12/2018 Solvay Process https://njit2.mrooms.net/mod/quiz/review.php?attempt=1152413&cmid=749635 2/8 Question 2 Correct Mark 1.00 out of 1.00 A weighed amount of sodium chloride is completely dissolved in a measured volume of 4.00 M ammonia solution at ice temperature, and carbon dioxide is bubbled in. Assume that sodium bicarbonate is formed until the limiting reagent is entirely used up. The solubility of sodium bicarbonate in water at ice temperature is 0.75 mol per liter. Also assume that all the sodium bicarbonate precipitated is collected and converted quantitatively to sodium carbonate The mass of sodium chloride in (g) is 11.11 The volume of ammonia solution in (mL) is 35.51 Calculate the following: How many moles of ammonia are present originally? Answer: 0.14204 Question 3 Correct Mark 1.00 out of 1.00 A weighed amount of sodium chloride is completely dissolved in a measured volume of 4.00 M ammonia solution at ice temperature, and carbon dioxide is bubbled in. Assume that sodium bicarbonate is formed until the limiting reagent is entirely used up. The solubility of sodium bicarbonate in water at ice temperature is 0.75 mol per liter. Also assume that all the sodium bicarbonate precipitated is collected and converted quantitatively to sodium carbonate The mass of sodium chloride in (g) is 11.11 The volume of ammonia solution in (mL) is 3 [Show More]
Last updated: 2 years ago
Preview 1 out of 8 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 29, 2022
Number of pages
8
Written in
Additional information
This document has been written for:
Uploaded
Mar 29, 2022
Downloads
0
Views
78