Chromatography.docx. Graded A+. Latest Comprehension
Document Content and Description Below
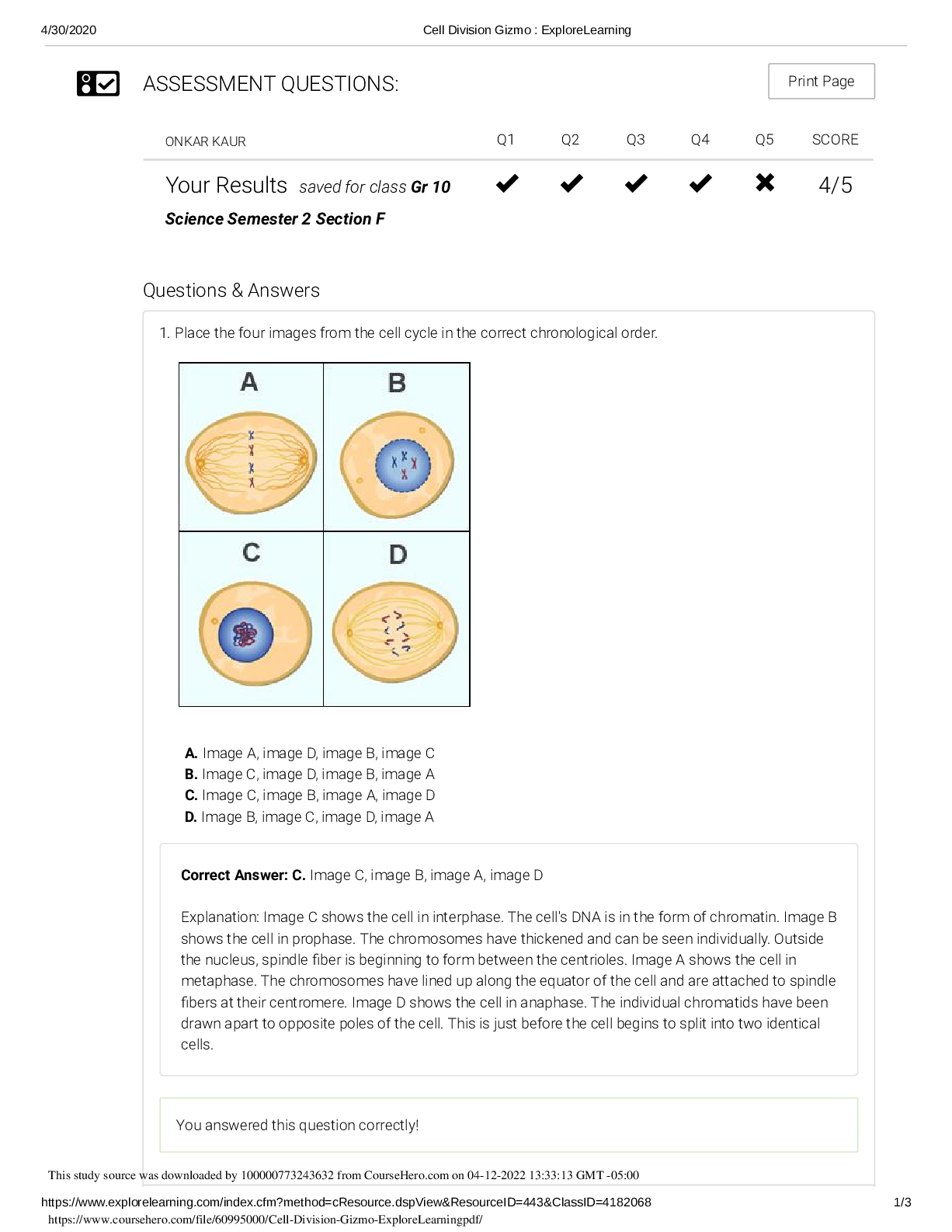
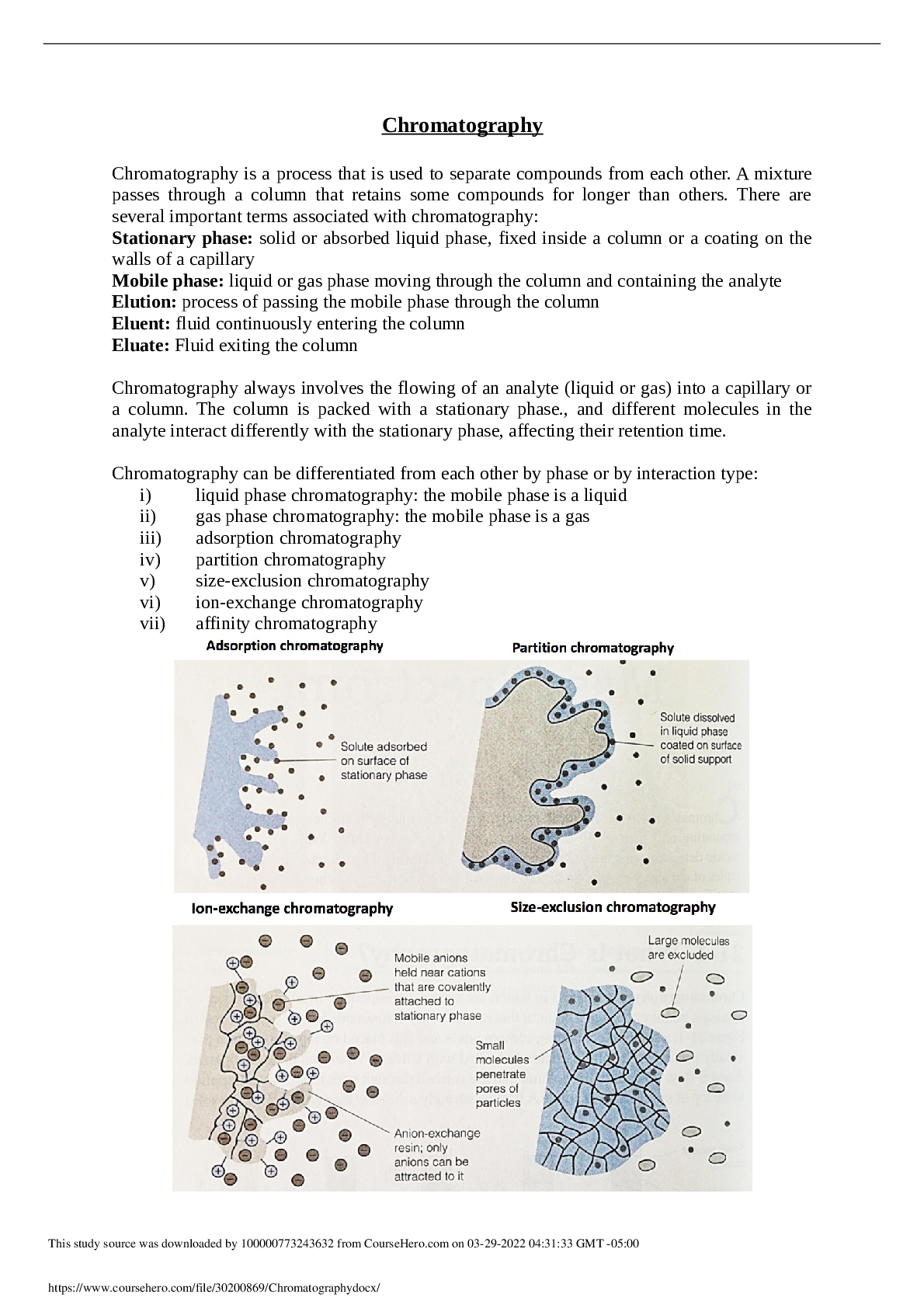
Chromatography is a process that is used to separate compounds from each other. A mixture passes through a column that retains some compounds for longer than others. There are several important term ... s associated with chromatography: Stationary phase: solid or absorbed liquid phase, fixed inside a column or a coating on the walls of a capillary Mobile phase: liquid or gas phase moving through the column and containing the analyte Elution: process of passing the mobile phase through the column Eluent: fluid continuously entering the column Eluate: Fluid exiting the column Chromatography always involves the flowing of an analyte (liquid or gas) into a capillary or a column. The column is packed with a stationary phase., and different molecules in the analyte interact differently with the stationary phase, affecting their retention time. Chromatography can be differentiated from each other by phase or by interaction type: i) liquid phase chromatography: the mobile phase is a liquid ii) gas phase chromatography: the mobile phase is a gas iii) adsorption chromatography iv) partition chromatography v) size-exclusion chromatography vi) ion-exchange chromatography vii) affinity ch [Show More]
Last updated: 3 years ago
Preview 1 out of 7 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$5.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 29, 2022
Number of pages
7
Written in
All
Additional information
This document has been written for:
Uploaded
Mar 29, 2022
Downloads
0
Views
95