Gizmos Student Exploration| Nuclear Reactions Answer Key 2020 [100% correct] [GRADED A+]
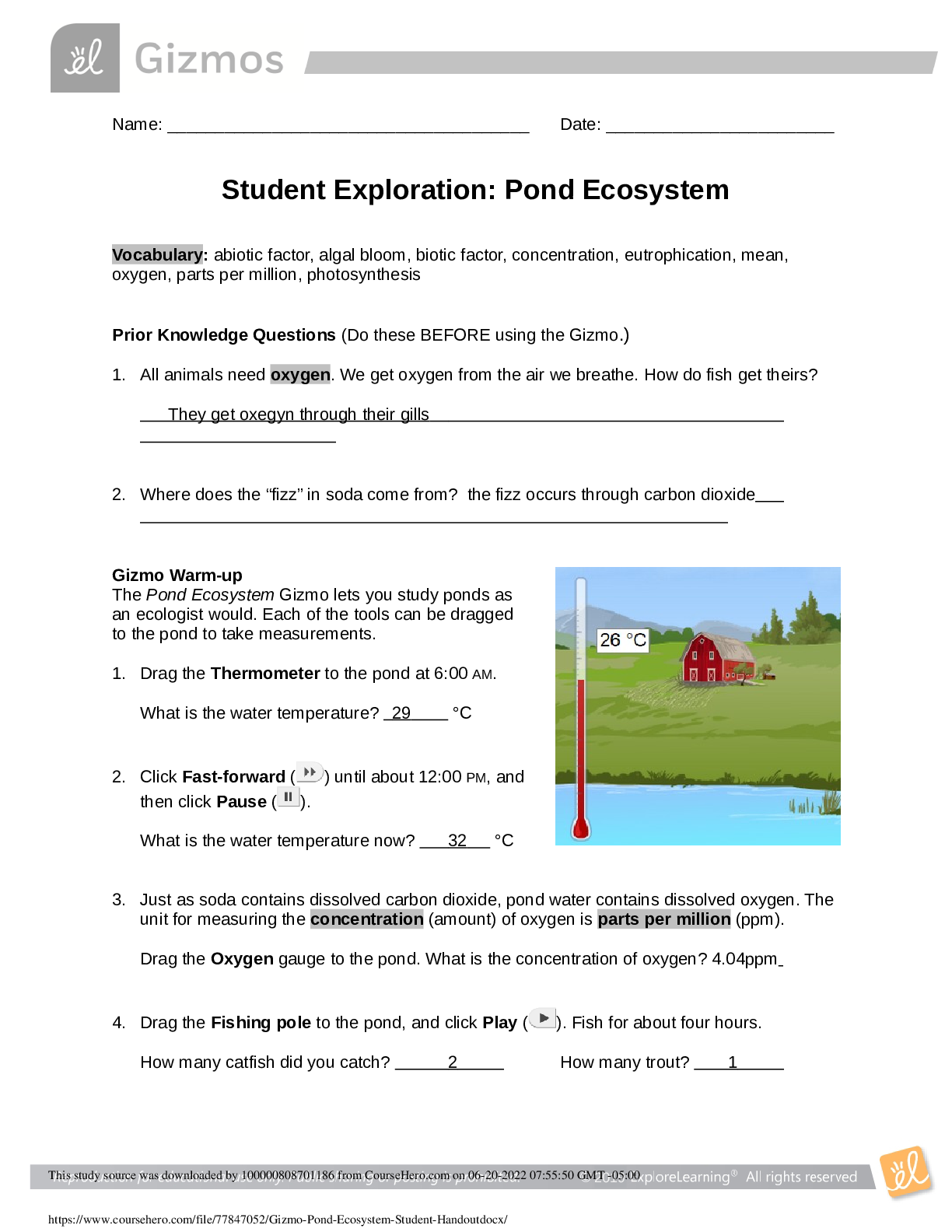
Document Content and Description Below
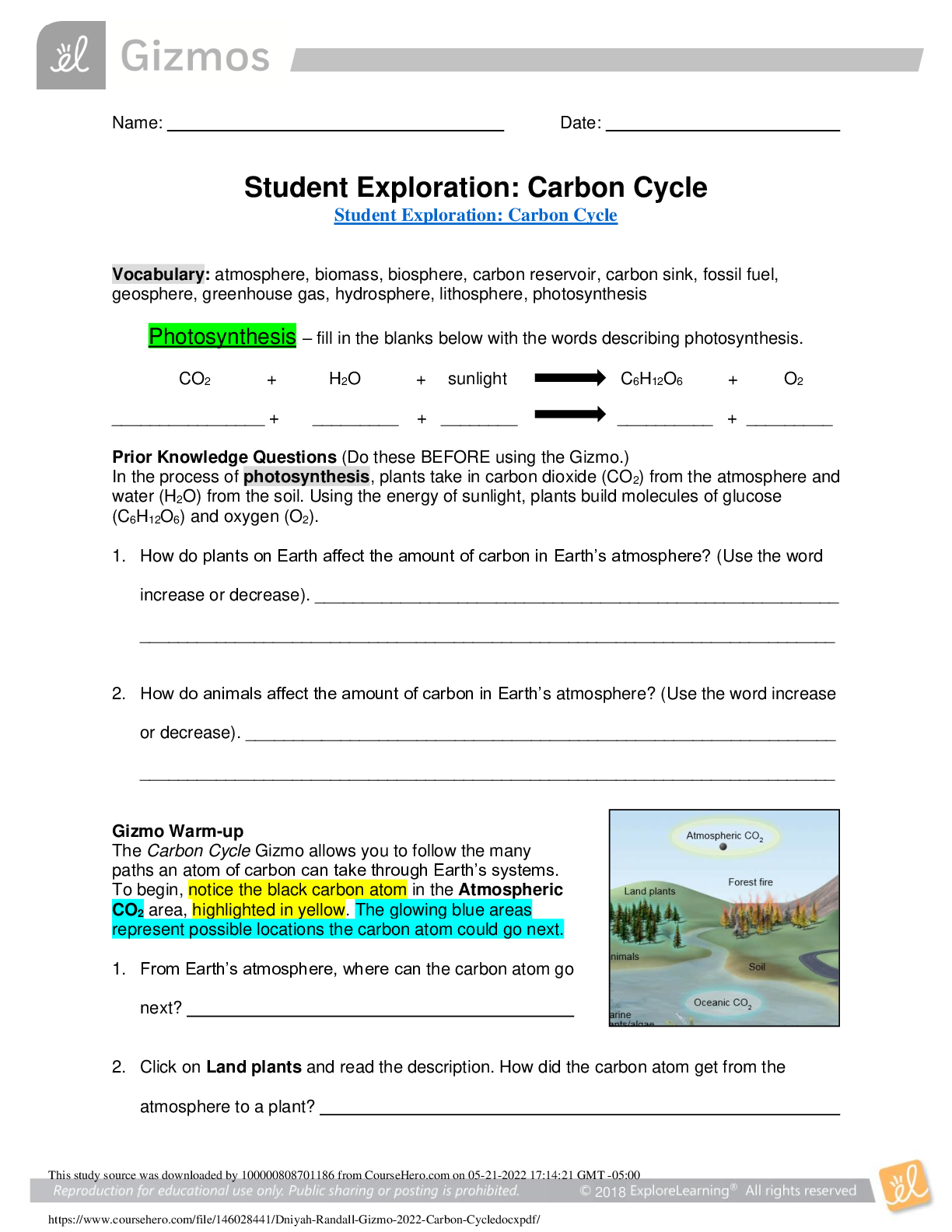
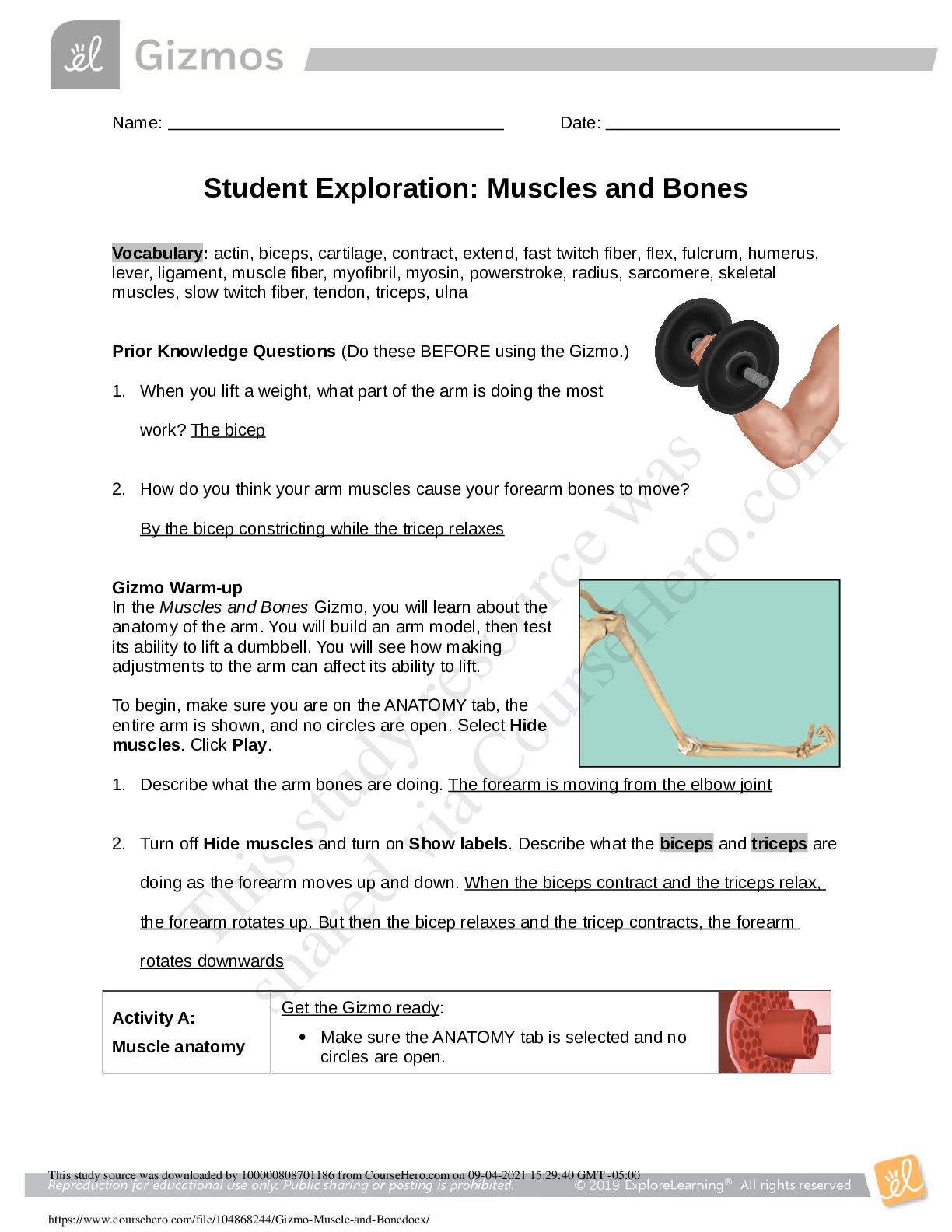
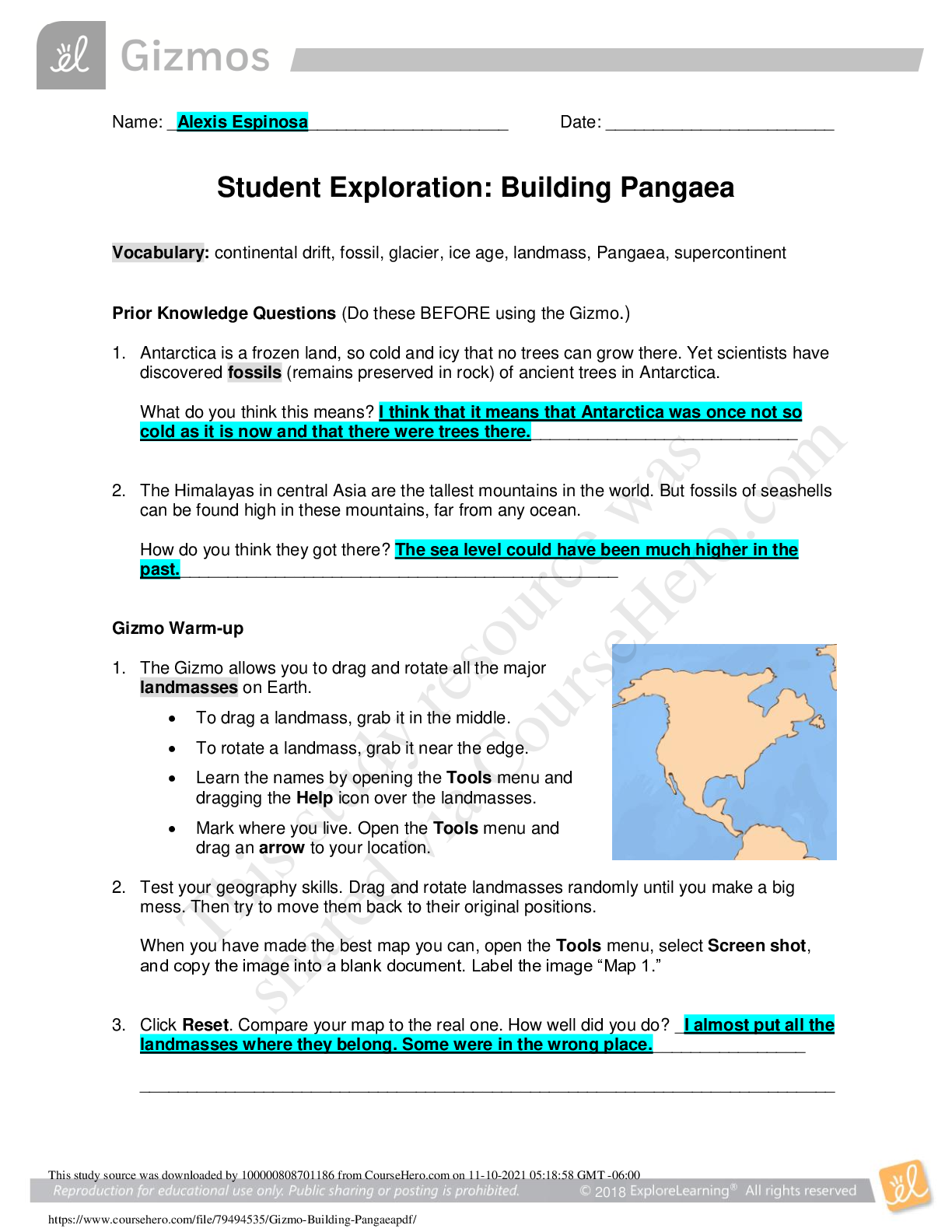
Student Exploration: Nuclear Reactions
[Note to teachers and students: This Gizmo was designed as a follow-up to the Nuclear Decay
Gizmo. We recommend doing that activity before trying this one.]
V
...
ocabulary: chain reaction, CNO cycle, catalyst, deuterium, electron volt, fission, fusion,
isotope, nuclear reaction, positron, positron emission, proton-proton chain
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
The chart to the right gives the isotope
name, element name, number of protons, and
number of neutrons of three isotopes.
1. What do you notice about the isotope
number and the sum of protons and
neutrons?
2. The element symbol for uranium-238 is . This means U-238 has a total mass of 238
and contains 92 protons. Write the element symbols for the isotopes in the table:
Hydrogen-1 Carbon-12 Uranium-235
Gizmo Warm-up
The Nuclear Reactions Gizmo simulates a particle accelerator.
Particle accelerators speed up atoms to very high velocities, then
crash the atoms together with enough energy to cause changes called
nuclear reactions. There are three particle beams available in this
Gizmo, protons, neutrons, and helium-3 nuclei.
1. Click Fire Proton to engage the first particle beam.
What happens?
2. Colliding particles don¶t alZa\s react. Click Reset, and then click Fire neutron.
A. Does a reaction occur?
B. Explain:
Isotope Protons Neutrons
Hydrogen-1 1 0
Carbon-12 6 6
Uranium-235 92 143
Alexa Nissim 6420
H E
No
The isotope number is equal to the sum of the protons and neutrons.
Proton joins nucleus
Neutron traveled through the nucleus
This study source was downloaded by 100000808701186 from CourseHero.com on 10-04-2021 22:28:54 GMT -05:00
https://www.coursehero.com/file/66386529/Nuclear-ReactionsSEpdf/
This study resource was
shared via CourseHero.com
2019
Activity A:
Proton-proton
chain
Get the Gizmo ready:
x Click Reset.
x Be sure Proton-proton is selected in the Reaction
menu.
Introduction: All stars turn hydrogen into helium in a process called nuclear fusion. Stars
perform this process in different ways. In stars like our sun, the proton-proton chain is used.
This reaction requires temperatures greater than 4,000,000 K to occur.
Question: How does the process of fusion turn hydrogen into helium in stars?
1. Observe: Click Fire proton and observe. What happens after the proton merges into the
nucleus?
This is a form of nuclear decay called positron emission. During positron emission, a
proton decays into a neutron. In this process, it emits a positron, which is a nearly massless
antimatter particle with a positive charge.
2. Observe: Click Reset and click Fire proton. Observe what happens. Many subatomic
particles appear frequently in nuclear reactions. Their element symbols are given below:
(Neutrinos are also produced but are beyond the scope of this Gizmo.)
Click Reset and click Fire proton. Turn on the Write equation checkbox. Based on what
you have observed, write in the equation for this reaction in the Gizmo and below.
A. Turn on Show equation. Was your predicted equation correct?
Correct your equation if necessary. The resulting H-2 isotope is called deuterium.
B. Emitted energy is reported in megaelectron volts (MeV), where one MeV is equal to
one million electron volts. How much energy is emitted in this reaction?
(Activity A continued on next page)
Neutron Positron Electron Proton
i H H 9 et
yes
1.44MeV
Proton converts to neutron, positron emitted
[Show More]
Last updated: 3 years ago
Preview 1 out of 7 pages




![Preview image of Gizmos Student Exploration| Nuclear Reactions Answer Key 2020 [100% correct] [GRADED A+] document](https://scholarfriends.com/storage/Nuclear_ReactionsSE.png)