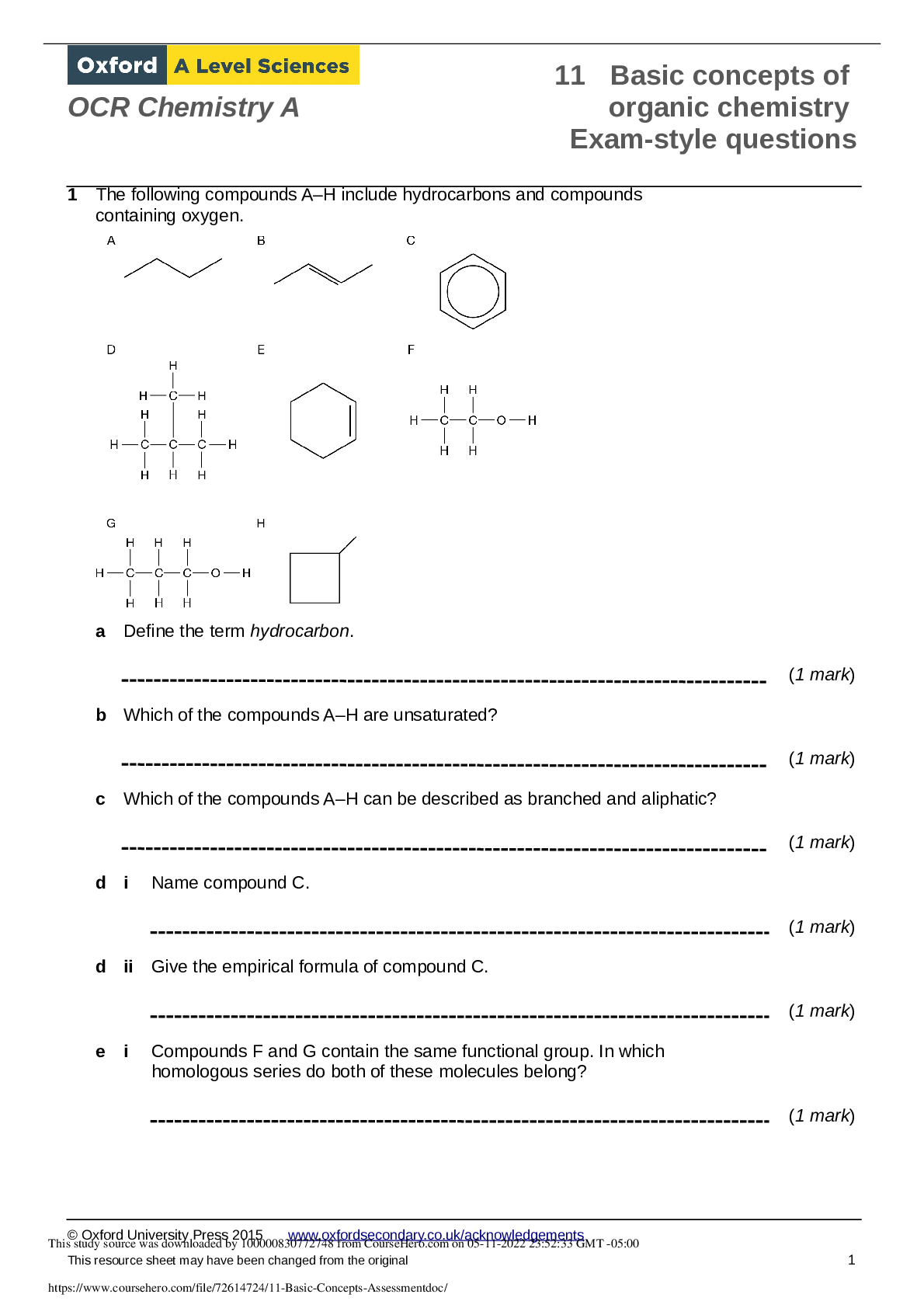
1 The following compounds A–H include hydrocarbons and compounds
containing oxygen.
a Define the term hydrocarbon.
(1 mark)
b Which of the compounds A–H are unsaturated?
(1 mark)
c Which of the compounds A–H can be
...
1 The following compounds A–H include hydrocarbons and compounds
containing oxygen.
a Define the term hydrocarbon.
(1 mark)
b Which of the compounds A–H are unsaturated?
(1 mark)
c Which of the compounds A–H can be described as branched and aliphatic?
(1 mark)
d i Name compound C.
(1 mark)
d ii Give the empirical formula of compound C.
(1 mark)
e i Compounds F and G contain the same functional group. In which
homologous series do both of these molecules belong?
(1 mark)
© Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 1
This study source was downloaded by 100000830772748 from CourseHero.com on 05-11-2022 23:52:33 GMT -05:00
https://www.coursehero.com/file/72614724/11-Basic-Concepts-Assessmentdoc/
11 Basic concepts of
organic chemistry
Exam-style questions
OCR Chemistry A
e ii Deduce the general formula for compounds F and G.
(1 mark)
f Give the molecular formula of compound H.
(1 mark)
g Are compounds A and B structural isomers? Explain your answer.
(1 mark)
2 There are three types of organic mechanism: addition, substitution and
elimination.
a Which type of organic mechanism will always have an atom economy of
100%? Explain your answer.
(1 mark)
b Classify each of the following reactions as either addition, substitution or
elimination:
i CH3CHBrCH3 NaOH → CH3CH(OH)CH3 NaBr
(1 mark)
ii CH3CHBrCH3 NaOH → CH2CHCH3 NaBr H2O
(1 mark)
iii Name the homologous series that the organic product from the reaction in
b ii belongs to.
(1 mark)
iv Draw the skeletal formula of the structural isomer of the organic product
in b ii and name it.
(2 marks)
© Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 2
This study source was downloaded by 100000830772748 from CourseHero.com on 05-11-2022 23:52:33 GMT -05:00
https://www.coursehero.com/file/72614724/11-Basic-Concepts-Assessmentdoc/
11 Basic concepts of
organic chemistry
Exam-style questions
OCR Chemistry A
3 Depending on the conditions used, the C–Br bond in the compound below can
break either homolytically or heterolytically.
a i When UV light is used, the C–Br bond fission is homolytic. Describe what
happens to the bonding electrons when the C–Br bond undergoes
homolytic fission.
(1 mark)
ii When the C–Br bond breaks homolytically, what name is given to the type
of particle produced?
(1 mark)
b i Name the type of particle produced when the C–Br bond breaks
heterolytically.
(1 mark)
ii Use curly arrows and partial charges to show the breaking of the C–Br
bond via heterolytic fission on the molecule drawn below.
(2 marks)
iii What does a curly arrow represent?
(1 mark)
4 Butanoic acid, shown below, is the chemical responsible for the distinctive smell
of human vomit.
a i Give the molecular formula of butanoic acid.
(1 mark)
ii State the homologous series in which butanoic acid belongs.
(1 mark)
© Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 3
This study source was downloaded by 100000830772748 from CourseHero.com on 05-11-2022 23:52:33 GMT -05:00
https://www.coursehero.com/file/72614724/11-Basic-Concepts-Assessmentdoc/
11 Basic concepts of
organic chemistry
Exam-style questions
OCR Chemistry A
b Butanoic acid is mainly used to prepare methyl butanoate, which is used in
perfumes as its fruity smell resembles that of pineapples. The equation for
this reaction is shown below:
CH3CH2CH2CO2H CH3OH → CH3CH2CH2CO2CH3 ?
Reaction 4.1
i Deduce the other product made in this reaction.
(1 mark)
ii State the homologous series in which methyl butanoate belongs and
draw the skeletal formula of this molecule.
(2 marks)
iii Suggest why methyl butanoate has a comparatively low boiling point
when compared to either of the reactants in Reaction 4.1 above.
(2 marks)
iv Suggest a reason, other than cost, why a perfume manufacturer would
choose to use the chemically manufactured pineapple fragrance rather
than the natural product.
(1 mark)
v After methyl butanoate has been produced via Reaction 4.1, it needs to
be purified. This is achieved by adding sodium carbonate. Suggest the
type of impurity removed when sodium carbonate is added and how you
would know when all of this impurity has been removed from the product.
(2 marks)
© Oxford University Press 2015 www.oxfordsecondary.co.uk/acknowledgements
This resource sheet may have been changed from the original 4
This study source was downloaded by 100000830772748 from CourseHero.com on 05-11-2022 23:52:33 GMT -05:00
https://www.coursehero.com/file/72614724/11-Basic-Concepts-Assessmentdoc/
11 Basic concepts of
organic chemistry
Exam-style questions
OCR Chemistry A
5 An organic compound, known to be a carbonyl, was analysed and found to
contain 66.7% carbon, 11.1% hydrogen and 22.2% oxygen by mass. Its
molecular mass was determined to be 72.
a Calculate the empirical formula.
(2 marks)
b Determine the molecular formula.
(1 mark)
c Draw and name all possible structures of this compound.
(6 marks)
©
[Show More]

 Latest Questions and Complete Solutions.png)

.png)
.png)