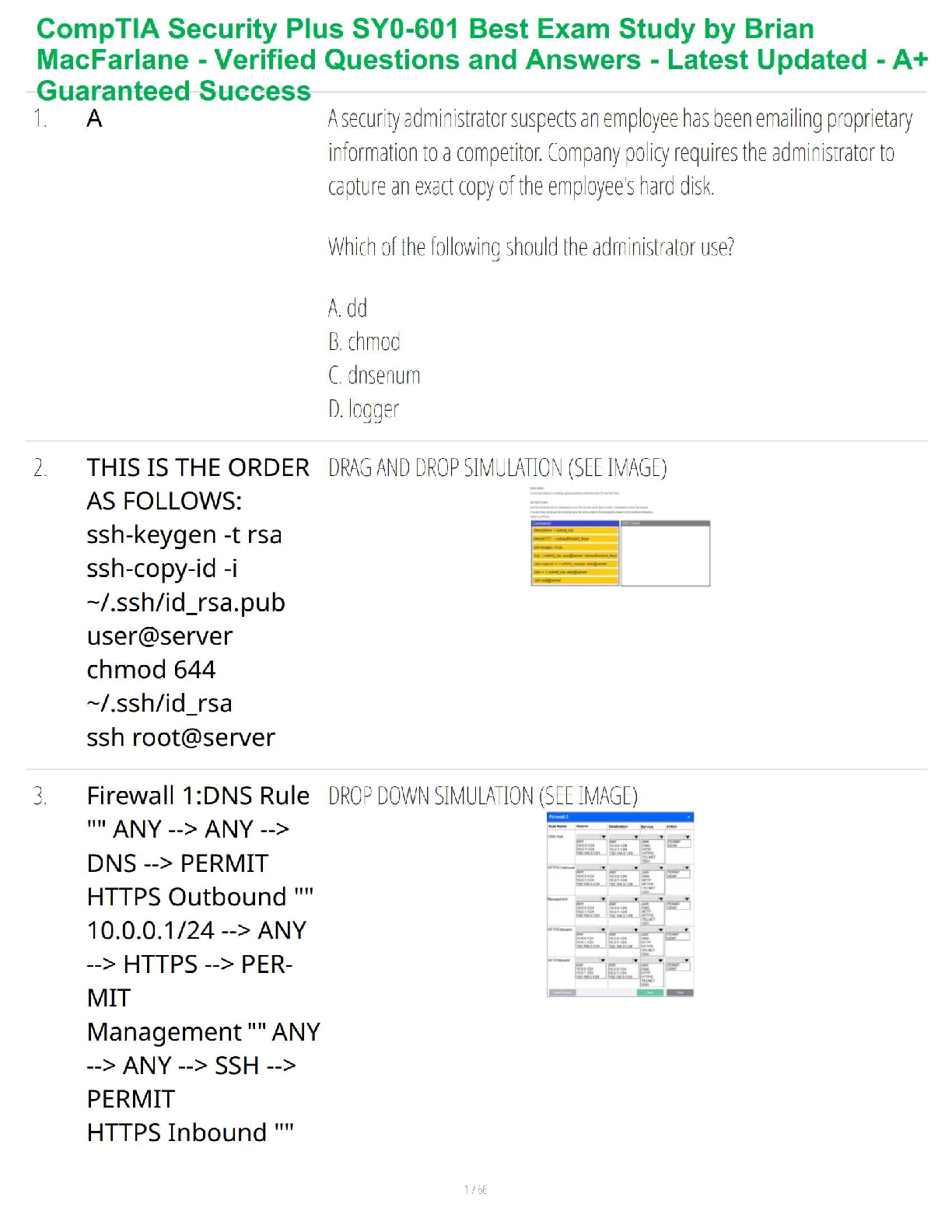
1. What is the basic structure of an amino acid? What do they look like?: -
amino group (NH2 or NH3), carboxyl group (COO or COOH), alpha carbon (C),
and variable group
2. How do you identify the 3 different types of
...
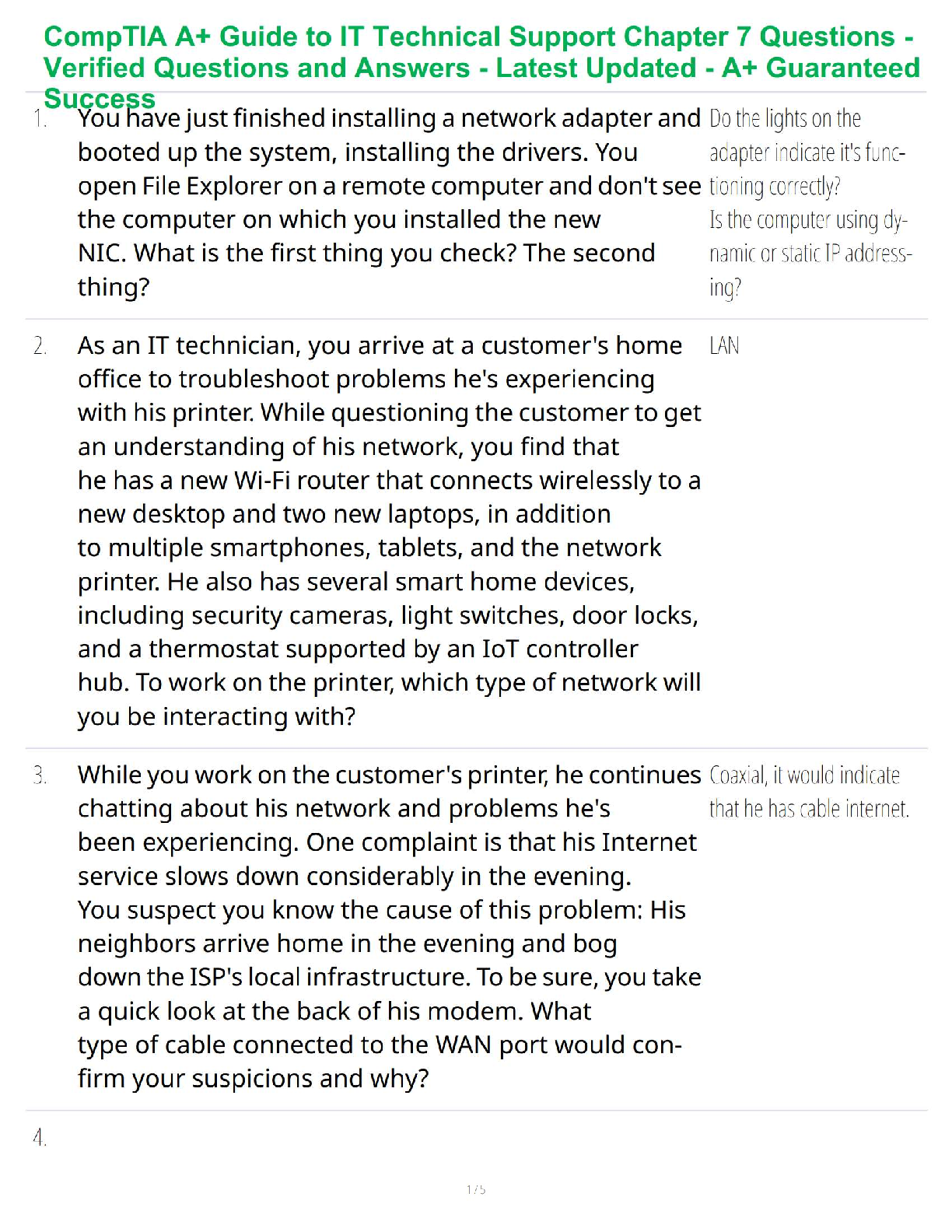
1. What is the basic structure of an amino acid? What do they look like?: -
amino group (NH2 or NH3), carboxyl group (COO or COOH), alpha carbon (C),
and variable group
2. How do you identify the 3 different types of side chains: non-polar/hydrophobic, polar, and charged?: Non-polar/hydrophobic - end with CH or "can't
have" water. Polar - end with OH, SH, or NH. Charged - end with a charge
3. what kinds of bonds do each of the 3 different types of side chains
make?: ionic, hydrophobic/non-polar, charged
4. What are the 4 levels of protein structure?: Primary - linear structure, Secondary - Folded into helix or pleated sheet caused by hydrogen bonding, tertiary -
3D structure caused by side chain interactions, quaternary - 1+ amino acid chains
combine = multiple subunits MUST have 1+ subunit
5. What enviormental change breaks each type of bond?: hydrophobic - temperature change, ionic - salt or decreased pH, hydrogen - temperature, change in
pH, disulfide - reducing agents
6. what type of amino acid side chain leads to protein aggregration?: hydrophobic bonds
7. how do environmental changes affect protein folding?: Extreme temp can
cause hydrogen bonds to break apart = malformation of protein folding
8. how do mutations affect protein structure?: Can cause structure to change.
Protein loses form = loses function. May form a different protein.
9. What is an electron?: Negatively charged atom on outer ring for bonding
10. What is energy:: Power derived fro chemical interaction
11. what are covalent bonds?: chemical bond, atoms share 1+ valence electrons
12. what is an ionic bond?: bond between positive and negative
13. what is a hydrogen bond?: weak bond between positive and negative
14. with an amino?: piece of amino acid, NH2 or NH3
15. what is a carboyxl?: piece of amino acid, COO or COOH
16. What is hydrophobic?: Doesn't like water, end with CH
17. what is hydrophilic?: Water Lovering, end with OH, NH, or SH
18. what is disulfide bond?: strongest bond between reduction agents, formed
between SH's.
WGU C785 FINAL EXAM QUIZ
GRADED A+
2 / 16
19. what are zwitterions?: amino with positive and negative charges = overall
charge of zero
20. what is a polypeptide: polymer of amino acids
21. What is dehydration synthesis?: Process of forming peptide bonds
22. what is hydrolysis?: adding water to destroy bonds
23. what is an alpha helix?: twisted secondary structure, formed by hydrogen
bonds
24. what is a beta sheet?: folded second structure shape, formed by hydrogen
bonds
25. what is denaturation?: loss of shape duet o interruption of chemical bonds;
occurs via extreme salt, temp, pH
26. what is aggregation?: clumping of inner or outer cellular proteins caused by
misfolded proteins leading to diseases such as Alzheimers, ALS, Parkinson's
27. how do enzymes catalyze reactions?: bind with substrates to decrease
activation energy required and decrease reaction rate
28. how do enzymes affect reaction rate and activation energy?: decrease
activation energy and decrease reaction rate
29. what are the 4 steps of the enzymatic cycle?: enzyme recognizes substrate, substrate attracts the enzyme; enzyme-substrate complex is formed; enzyme-product complex formed; product is released, enzyme recycled
30. how do environmental changes affect enzymes?: High heat, pH change,
high salt concentration, and reducing agents can cause an enzyme to lose its
form/lose function
31. what is a competitive inhibitor?: Mimics substrate and takes its place on the
active binding site
32. what is a noncompetitive inhibitor?: Binds to allosteric site causing active
site to change shape = preventing substrate from binding with enzyme
33. what molecules increase/build up or decrease given a specific inhibitor?
A -> (enzyme 1) -> B -> (enzyme 2) -> C -> (enzyme 3) -> D. Pretend Enzyme 2
is inhibited.: Inhibitor would cause a build up for product B, decrease product C.
Enzyme 3 and product D would not be created.
34. what is substrate?: the substance on which an enzyme acts
35. what is a product?: result of a reaction
WGU C785 FINAL EXAM QUIZ
GRADED A+
3 / 16
36. what is an intermediate?: products produced in an enzyme pathway before
final product
37. what is an active site?: location where substrate binds with enzyme
38. what is enzyme specificity?: Enzymes bind with certain substrate or type of
substrate to create a certain reaction
39. what is induced fit?: Enzyme changes shape in enzyme-substrate complex
to facilitate formation of enzyme-product complex
40. what is kinase?: Enzyme, adds phosphate group via phosphorlation
41. what is phosphatase?: enzyme, removes phosphate group via dephosphorylation
42. with is an allosteric site?: secondary site on an enzyme an inhibitor binds to
via non-competitive inhibition
43. what is competitive inhibition?: enzyme substrate and inhibitor complex
compete to bind with enzyme's active site. no product formed when inhibitor binds
with enzyme.
44. what is non-competitive inhibition?: inhibitor binds to allosteric site, not
active site. Changes shape of active site, preventing substrate from binding and
making product
45. what is feedback inhibition?: End product sends feedback to beginning of
enzyme pathway inhibiting 1st enzyme via noncompetitive inhibition
46. what nucleotides/bases are used in DNA? what are their abbreviations/full names?: C - cytosine, G - guanine, A - adenine, T - thyamine
47. what nucleotides/bases are used in RNA?: C - cytosine, G - guanine, U -
uracil, A - adenine
48. which nucleotides base-pair together in DNA?: T-A, G-C
49. which nucleotides base-pair together in RNA?: U-A, G-C
50. how to we make complementary DNA? (i.e. coding to temple et reverse)-
: Taking coding DNA, write in reverse, then pair them up to make template.
Template DNA, write in reverse, then pair up to make coding
51. how do we make mRNA?: template DNA to mRNA by switching back and
forth OR coding DNA to mRNA by switching out T's for U's
52. which strand of DNA is complementary to mRNA?: Template DNA
WGU C785 FINAL EXAM QUIZ
GRADED A+
4 / 16
53. how do we make protein?: DNA -> RNA -> Protein
54. which type of nucleotide sequence is used and in which direction?: RNA
is used 5' to 3'
55. what is the relationship between mRNA and tRNA?: tRNA is complementary to mRNA
56. how does mRNA splicing allow use to create multiple proteins from a
single gene/mRNA?: Alternative splicing allows for all
[Show More]











.png)
.png)