Gizmo Warm-up Student Exploration- Density Laboratory 2
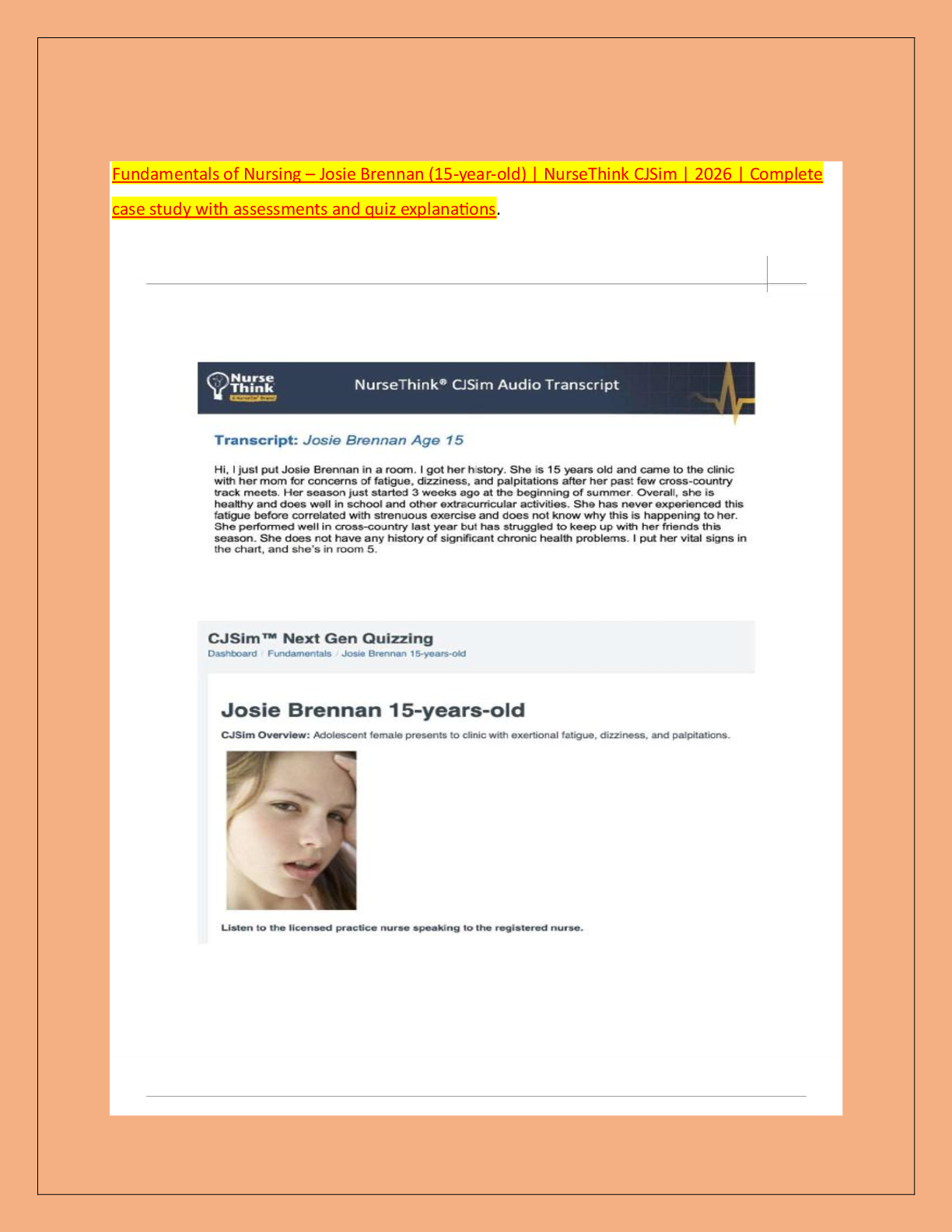
Document Content and Description Below
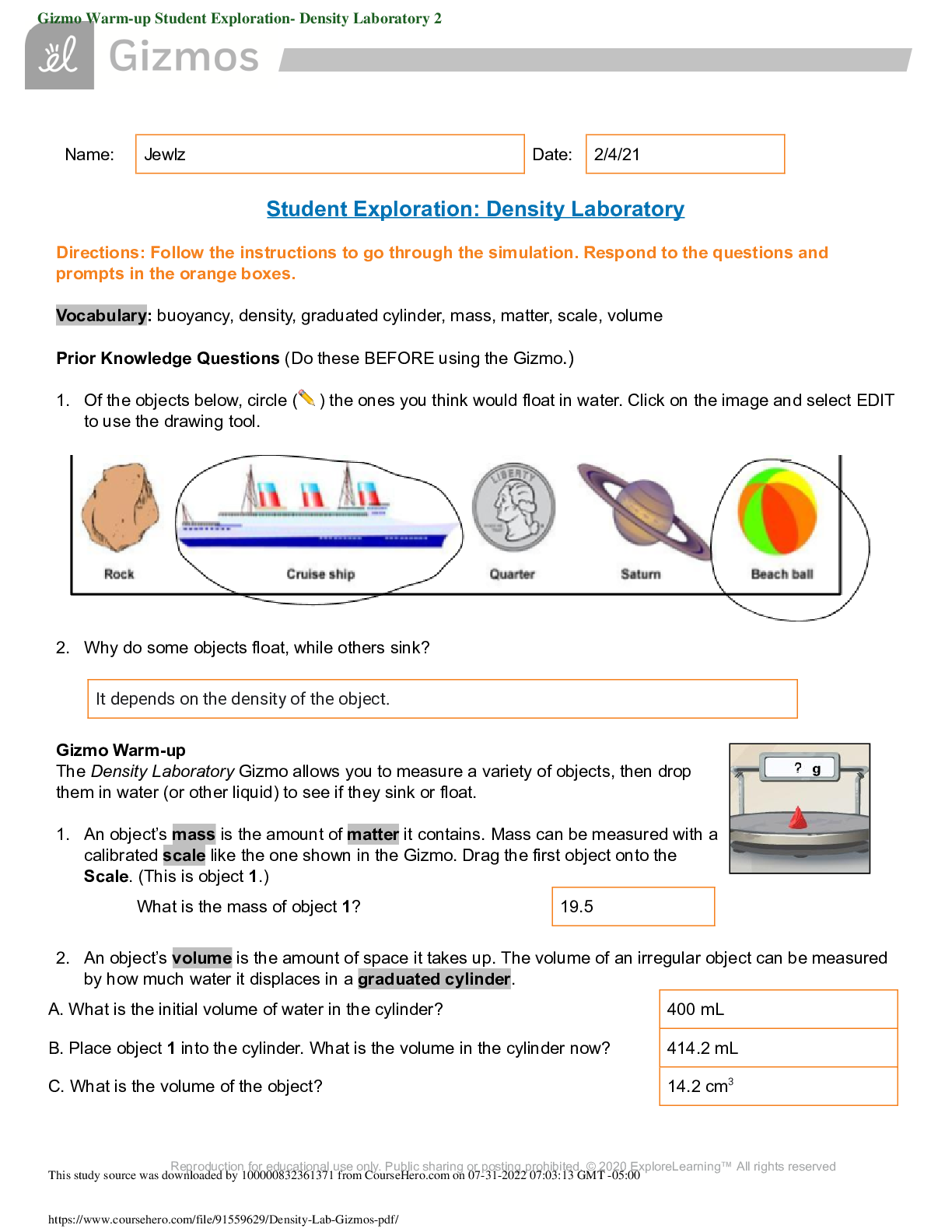
Student Exploration: Density Laboratory Directions: Follow the instructions to go through the simulation. Respond to the questions and prompts in the orange boxes. Vocabulary: buoyancy, density, gr ... aduated cylinder, mass, matter, scale, volume Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. Of the objects below, circle (✏ ) the ones you think would float in water. Click on the image and select EDIT to use the drawing tool. 2. Why do some objects float, while others sink? Gizmo Warm-up The Density Laboratory Gizmo allows you to measure a variety of objects, then drop them in water (or other liquid) to see if they sink or float. 1. An object’s mass is the amount of matter it contains. Mass can be measured with a calibrated scale like the one shown in the Gizmo. Drag the first object onto the Scale. (This is object 1.) 2. An object’s volume is the amount of space it takes up. The volume of an irregular object can be measured by how much water it displaces in a graduated cylinder. Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved Name: Jewlz Date: 2/4/21 It depends on the density of the object. What is the mass of object 1? 19.5 A. What is the initial volume of water in the cylinder? 400 mL B. Place object 1 into the cylinder. What is the volume in the cylinder now? 414.2 mL C. What is the volume of the object? 14.2 cm3 This study source was downloaded by 100000832361371 from CourseHero.com on 07-31-2022 07:03:13 GMT -05:00 https://www.coursehero.com/file/91559629/Density-Lab-Gizmos-pdf/ Note: While milliliters (mL) are used to measure liquid volumes, the equivalent unit cubic centimeters (cm3 ) is used for solids. Therefore, write the volume of object 1 in cm3 . Question: How can you predict whether an object will float or sink? 1. Observe: Experiment with the different objects in the Gizmo. Observe the mass and volume of each object, then drag it into the Beaker of liquid to see if it floats or sinks. (Notice a pin holds objects in the graduated cylinder, whether they sink or float.) Try to determine what the floating objects have in common and what the sinking objects have in common. 2. Form hypothesis: Compare the floating objects, then do the same for the sinking objects. 3. Collect data: Measure the mass and volume of objects 1 through 12, and record whether they float or sink in the table below. Leave the last column blank for now. Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved Activity A: Float or sink? Get the Gizmo ready: ● Drag object 1 back to the shelf. ● Check that Liquid density is set to 1.0 g/mL. A. What do the floating objects have in common? Objects that float have a lower density than the substance it is in. B. What do the sinking objects have in common? Objects that float have a higher density than the substance it is in. Object Mass (g) Volume (cm3 ) Float or sink? 1 19.5 14.2 sink 2 11.1 9.7 sink 3 4.3 5.6 float 4 134.8 7.8 sink 5 3.9 3.5 sink 6 78.7 29.2 sink 7 2.3 20.8 float 8 24.4 25.7 float 9 99.6 43.9 sink 10 42.1 60.8 float 11 65.5 41.2 sink 12 104.3 114.1 float This study source was downloaded by 100000832361371 from CourseHero.com on 07-31-2022 07:03:13 GMT -05:00 https://www.coursehero.com/file/91559629/Density-Lab-Gizmos-pdf/ 4. Analyze: Look carefully for patterns in your data. 5. Calculate: The density of an object is its mass per unit of volume. Dense objects feel very heavy for their size, while objects with low density feel very light for their size. To calculate density, divide the mass by volume: D = m/V. If the mass is measured in grams and volume in cubic centimeters, the unit of density is grams per cubic centimeter (g/cm3 ). Calculate the density of each object, and record the answers in the last column of your data table. Label this column “Density (g/cm3 ).” 6. Analyze: Compare the density of each object to the density of the liquid, 1.0 g/mL. This is the density of water. 7. Draw conclusions: If you know the mass and volume of an object, how can you predict whether it will float or sink in water? Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved A. Does mass alone determine whether an object will float or sink? Explain: No, you need both mass and volume to find its density to see if it can float. B. Does volume alone determine whether an object will float or sink? Explain: No, you need both mass and volume to find its density to see if it can float. C. Compare the mass and volume of each object. What is true of the mass and volume of all the floating objects? The volume was more than the mass of each object. D. What is true of the mass and volume of all the sinking objects? The mass is larger than the volume. A. What do you notice about the density of the floating objects? The object will float if it is less dense than the liquid it is placed in. B. What do you notice about the density of the sinking objects? The object will sink if it is denser than the liquid it is placed in. One can predict whether or not an object will float or sink in water by calculating its density by dividing the mass of the object by the volume of the object. If the density is more than one, the object will sink in water, but if the density is less than one, it will float. [Show More]
Last updated: 3 years ago
Preview 1 out of 5 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jul 31, 2022
Number of pages
5
Written in
All
Additional information
This document has been written for:
Uploaded
Jul 31, 2022
Downloads
0
Views
61