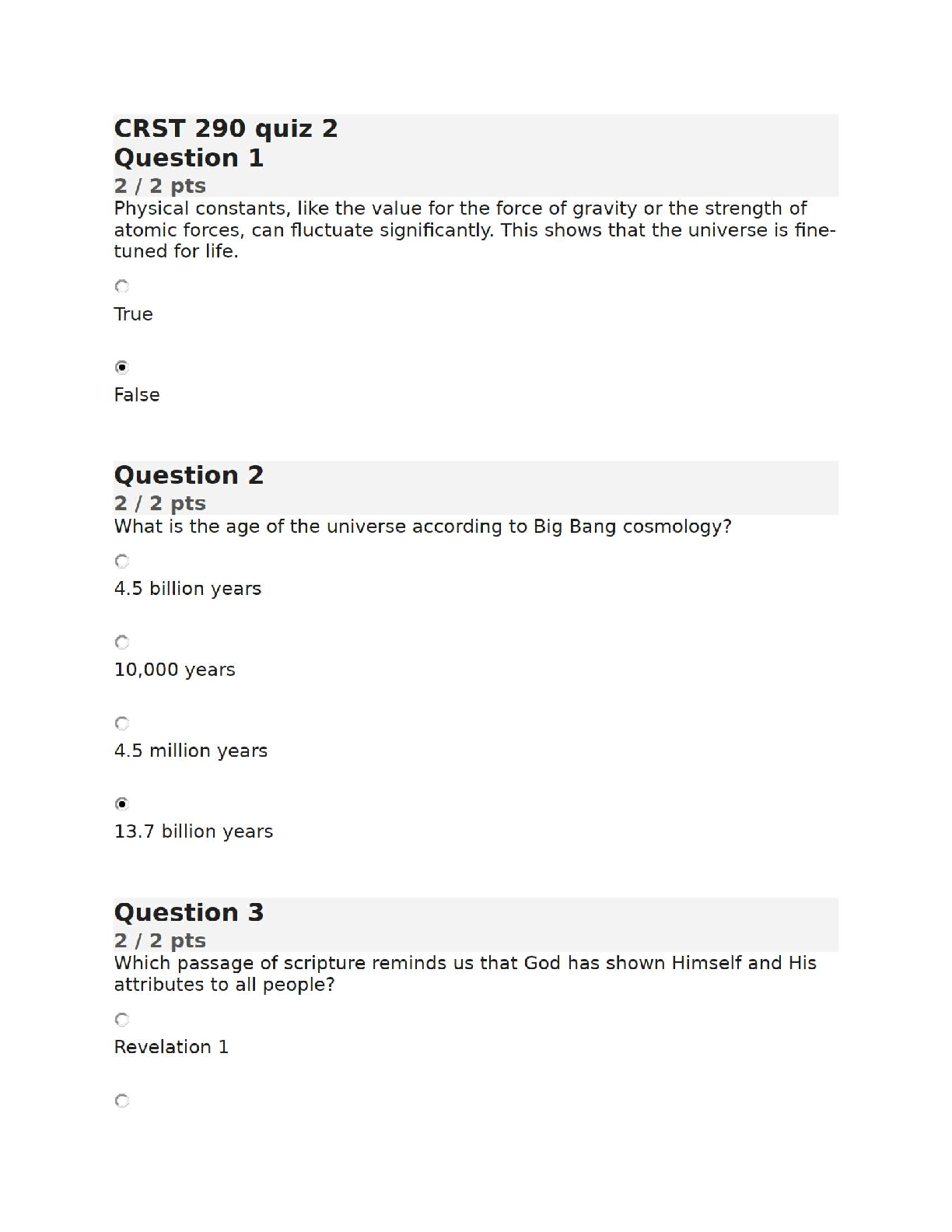
CRST 290 (History of Life) – Quiz 2 | Liberty University | Verified Answers & Topic Breakdown
Health Care > EXAM > CITI Training Test Prep | 134 Questions with 100% Correct Answers (All)
What must you file before conducting human clinical trials with an experimental drug? - ✔✔IND application (Form FDA 1571) During the clinical development phase of the IND process, what must spon ... sors do? - ✔✔Maintain current IND application by amending IND with new Form FDA 1571 and providing FDA with: safety updates copies of new protocols FDA 1572 Annual Progress reports this document notifies FDA of relevant changes in investigators conducting clinical trials under the IND. - ✔✔Form FDA 1572 What is the timeline of drug development? - ✔✔Preclinical trials, IND Submission, Clinical Development (Phase I-III), NDA submission, Marketing (Phase IV) When does a sponsor submit the IND? - ✔✔Prior to clinical development phases (human trials). *30 day process. What is the NDA? - ✔✔New Drug Application, submitted prior to Phase IV marketing phase. How long does the NDA submission take? - ✔✔6 months - 2 years. What is determined for a drug in the preclinical phase - ✔✔Pharmacokinetics and bioavailability Outcome shows promise of safety and efficacy warranting additional studying. How long does FDA have to review IND submission? - ✔✔30 days How long is the clinical development phase (Phase 1-III) in process for IND? - ✔✔6-7 years. [Show More]
Last updated: 3 years ago
Preview 1 out of 13 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:


Bundle for CITI Certification Tests Compilation
By Tessa 3 years ago
$29
8
Can't find what you want? Try our AI powered Search
Connected school, study & course
About the document
Uploaded On
Aug 12, 2022
Number of pages
13
Written in
All
This document has been written for:
Uploaded
Aug 12, 2022
Downloads
0
Views
181
Scholarfriends.com Online Platform by Browsegrades Inc. 651N South Broad St, Middletown DE. United States.
We're available through e-mail, Twitter, Facebook, and live chat.
FAQ
Questions? Leave a message!
Copyright © Scholarfriends · High quality services·