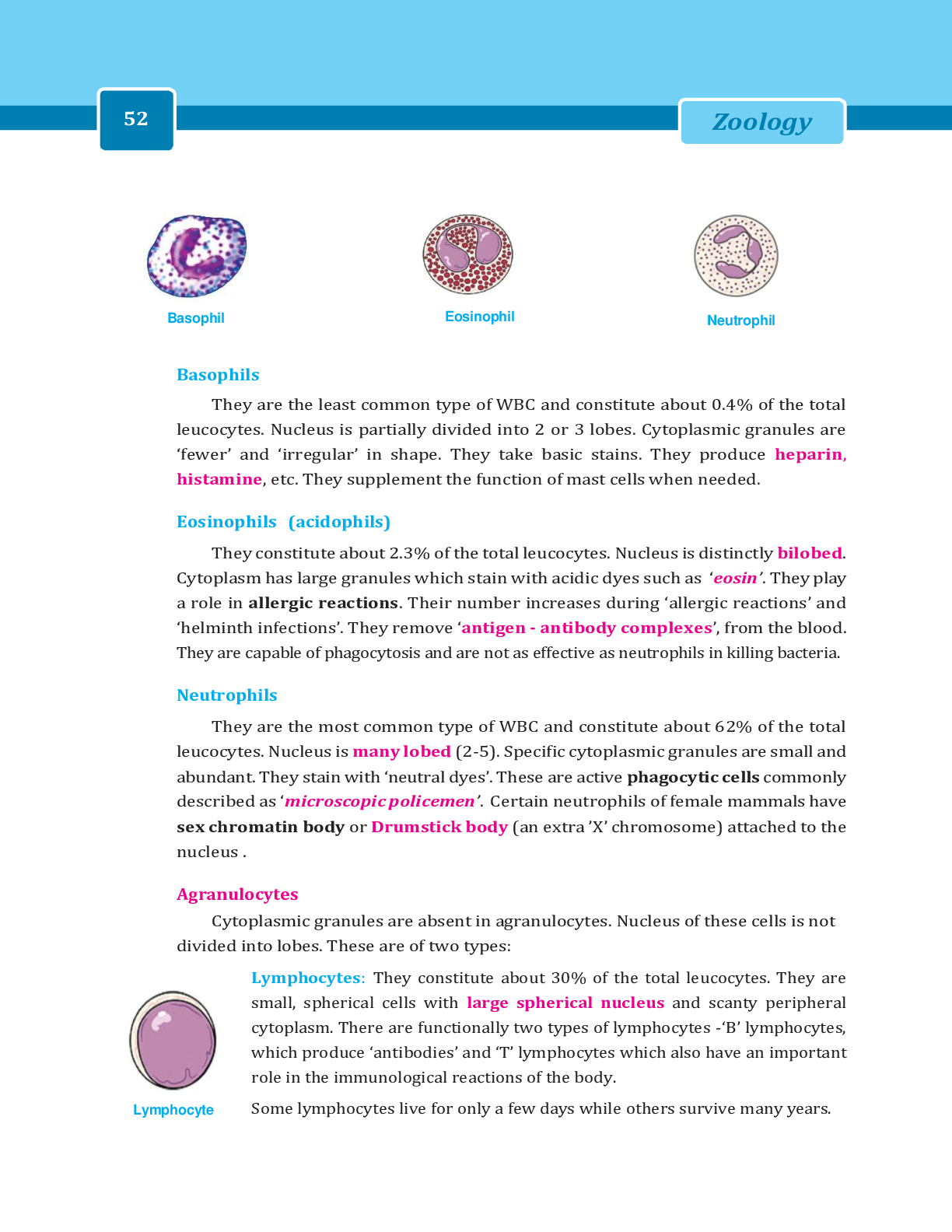
*NURSING > LECTURE SLIDES/NOTES > Principles and Practice of Clinical Trial Medicine (All)
Principles and Practice of Clinical Trial Medicine
Document Content and Description Below
While we may not recognize it, we all use the skills necessary to conduct and interpret “ clinical trials ” every single day. Sampling and comparing one restaurant, article of clothing, televis ... ion show, fi tness club, vacation location, date, job candidate, or client to another is effectively conducting a clinical trial. Evidence and results from these mini-trials guide your choices and decisions throughout the day. Should you buy that swimsuit? Is it better than the one in the other store? Are the Philadelphia Eagles or the Pittsburgh Steelers a better football team? Which sushi restaurant has the best salmon? What job will provide the best experience? Where should you live and what house should you buy? Is it better to hire Job Candidate #1 or Job Candidate #2? Faulty “ trial ” design, data, or interpretation leads to inaccurate assessments and perhaps poor decisions. For example, using the wrong criteria will result in hiring the wrong person for a job. We all have suffered from that impulse buy, that forehead-slapping wrong decision, or that bad choice of friend, employee, or signifi cant other. Formal and informal clinical trials are a large part of our lives. If you use, produce, study, purchase, invest in, or conduct research in drugs, medical devices, or any type of health care intervention, understanding the science and operations of formal clinical trials can only help. Today, even understanding many major news items requires at least some knowledge of clinical trials. Whenever a drug or medical device is recalled, a medical intervention is debunked, or a new therapy hits the market, clinical trial design, conduct, or analysis is at the heart of the evidence or the controversy. Health care is such a major business that even seemingly unrelated industries and professions can be dramatically affected by a successful or unsuccessful clinical trial. Flaws in a clinical trial that force a major drug or device to be pulled from the market can alter many lives and rock the economy. Therefore, during our planning stages for Principles and Practices of Clinical Trial Medicine , confi ning the book ’ s audience was diffi cult. Should this book be geared toward just physicians? Pharmaceutical industry professionals? Statisticians? Academics? Clinical research specialists? Regulatory professionals? Ethicists? Medical students? Nursing students? Medicine residents? Graduate students? Post-doctoral fellows? Epidemiologists? Engineers? Pharmacologists? Pharmacists? Biologists? Pharmaceutical or medical device executives? The more we thought about it, the more we realized that the audience could be quite broad. Both of our career journeys have taken us through a variety of functions and domains in industry, academics, and business. We have seen the investment, research, technical, management, teaching, writing, consulting, and clinical practice realms of the health care industry. In the end, while each area may have different jargon, cultures, personalities, and perspectives, the guiding principles are the same. A good clinical trial at an academic institution is a good one in industry and vice-versa. Therefore, we wrote this book with a broad audience in mind, trying to minimize the jargon and explain any important terminology in the process. The goal was to write a book that could be easily understood regardless of your background, especially since people from so many different backgrounds are involved in clinical trials. In fact, in many professions, understanding the jargon and terminology is half the battle. Moreover, regardless of your interest and function in the clinical research world, knowing the general concepts of all aspects of clinical trials can be very advantageous. In many ways, the clinical research world has become far too specialized. Many individuals stay ensconced within their areas of knowledge and expertise. But the best clinical researchers or trialists have broad knowledge bases that span statistics, regulatory affairs, ethics, clinical medicine, science, basic probability, data management, and trial and personnel management. The ones that stand out, are most marketable, and do the best work cannot afford to say, “ I do not need to know that because it is not in my area. ” xii Introduction Designing, conducting, and analyzing a clinical trial is like designing, building, and using a house. Recognizing a house ’ s design and construction helps you realize its potential use. For example, a thin-walled house may cause problems during the winter. Very cramped rooms may not facilitate hosting a party. At the same time, anticipating the house ’ s use aids its design and construction. Your design of a beach house likely will differ signifi cantly from your design of a farm house or a city dwelling. The “ building a house ” analogy helps illustrate the general organization of our book. The Principles and Practices of Clinical Trial Medicine contains fi ve sections. Section I introduces the fi eld of clinical research with Chapter 1 delineating some general theory and Chapter 2 covering important legal, ethical, and regulatory issues. The materials in this section are analogous to all of the rules and regulations that govern the construction of a house: ranging from general engineering and architectural principles to zoning laws and building codes. Just as you can ’ t build any kind of house anywhere you choose (e.g., Igloos do not belong in Philadelphia or San Francisco), you must understand general clinical research theory and comply with legal, ethical, and regulatory principles when designing and conducting a trial. Section II focuses on the general design of clinical trials. If you imagine a clinical trial to be a house, statistics (Chapter 3) are the tools used to build the house. The fi nal design of the house depends heavily on the tools that you have at your disposal. Sure you can rely on others to choose and wield the tools… but would you truly know and trust the house? To be truly competent at clinical research, you have to know your tools, even if you have specialists to employ them. Measures and Variables (Chapter 4) are the construction materials for the house. Construction materials help determine the house ’ s appearance and utility. Building a house resistant to harsh elements may be diffi cult without good quality bricks or cinder blocks. Similarly, studying heart disease may be challenging without accurate echocardiograms, electrocardiograms, and blood pressure measurements. Study Groups (Chapter 5) and Periods, Sequences and Design (Chapter 6) are the rooms and corridors of the house. Changing these will dramatically change the house ’ s functionality and purpose. Having no kitchen makes cooking and hosting dinner parties diffi cult. An indoor garage allows you to shield your car from the elements. Similarly, comparing two medical interventions normally requires employing at least two different study groups. Seeing the long-term effects of a drug necessitates patients being on the drug for a long period of time. Section III takes a closer look at an array of important elements in clinical trial design. Endpoints (Chapter 7) are special measures and variables that serve as the outcomes of the trial. So, continuing our building analogy, endpoints are the key construction materials that determine the worth, strength, and use of the house. Chapter 8 (Economics and Patient Reported Outcomes) discusses some special types of endpoints, Chapter 9 (Patient Selection and Sampling) reviews considerations when choosing patients for your trial, and Chapter 10 (Dosing and Intervention) analyzes how medical interventions should be administered to patients. All of these are as important factors and parameters to clinical trials as ceiling height, room size, lighting, house temperature, and other features are to house construction. Chapter 11 Epidemiology, Decision analysis and simulation offers additional tools that may help in the planning and analysis of trials, and is analogous to the model building and “ roughing-in ” phase of house building, where you visualize how a house might look. Section IV covers practical logistical issues involved in conducting a trial. This is analogous to concerns that arise when actually building the house. For example, from where do you procure building materials? Which forms should you complete when ordering such materials? Which nails should you use? Where should you place the beams? How do you select and supervise the contractor? All of these types of issues are discussed in Chapter 12 Study Execution. Recruiting Patients and Choosing Trial Locations (Chapter 13) are such an important part of conducting trials that a separate chapter is devoted to the topic. Finally, Section V discusses how to analyze the results of clinical trial. In our building analogy, this is similar to using and inhabiting the house. Data is the output of a clinical trial, just as a house is the end product of house construction. Chapter 14, Assessing Data Quality and Transforming Data, is akin to inspecting the house and making the fi nal adjustments and reworking anything that needs to be reworked. If the stairs are not to code, they need to be redone, and if the painters overpainted the moldings, they need to be repainted. Data similarly need to the cleaned and transformed, to ameliorate missing or unreliable data points. Chapter 15, Analysis of Data, is akin to decorating the house and moving the furniture into the appropriate rooms. You manipulate the data that has been gathered and prepared. This allows you to then interpret the data, which is the subject of Chapter 16 Data interpretation and conclusions. This is akin to moving into the house and living in it. This is the acid test. No matter how well-built or well-decorated the house is, if you don ’ t enjoy living in it, all has been for naught. Similarly, the ultimate end product of a clinical trial is a conclusion that is actionable for the treatment of future patients. So whether you are new to the world of clinical trials or have been conducting clinical research for many years, we hope that this book serves you well. The importance and use of clinical trials will only continue to grow in the future. Concomitantly, trial design and conduct will face increasing scrutiny. In many cases, lives of innumerable patients and signifi cant amount of time and resources will be riding on them. Will you be ready? [Show More]
Last updated: 3 years ago
Preview 1 out of 536 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$23.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 09, 2022
Number of pages
536
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 09, 2022
Downloads
0
Views
135