Chemistry > Lab Experiment > Isotope_of_Beanium_Lab | Download for quality grades (All)
Isotope_of_Beanium_Lab | Download for quality grades
Document Content and Description Below
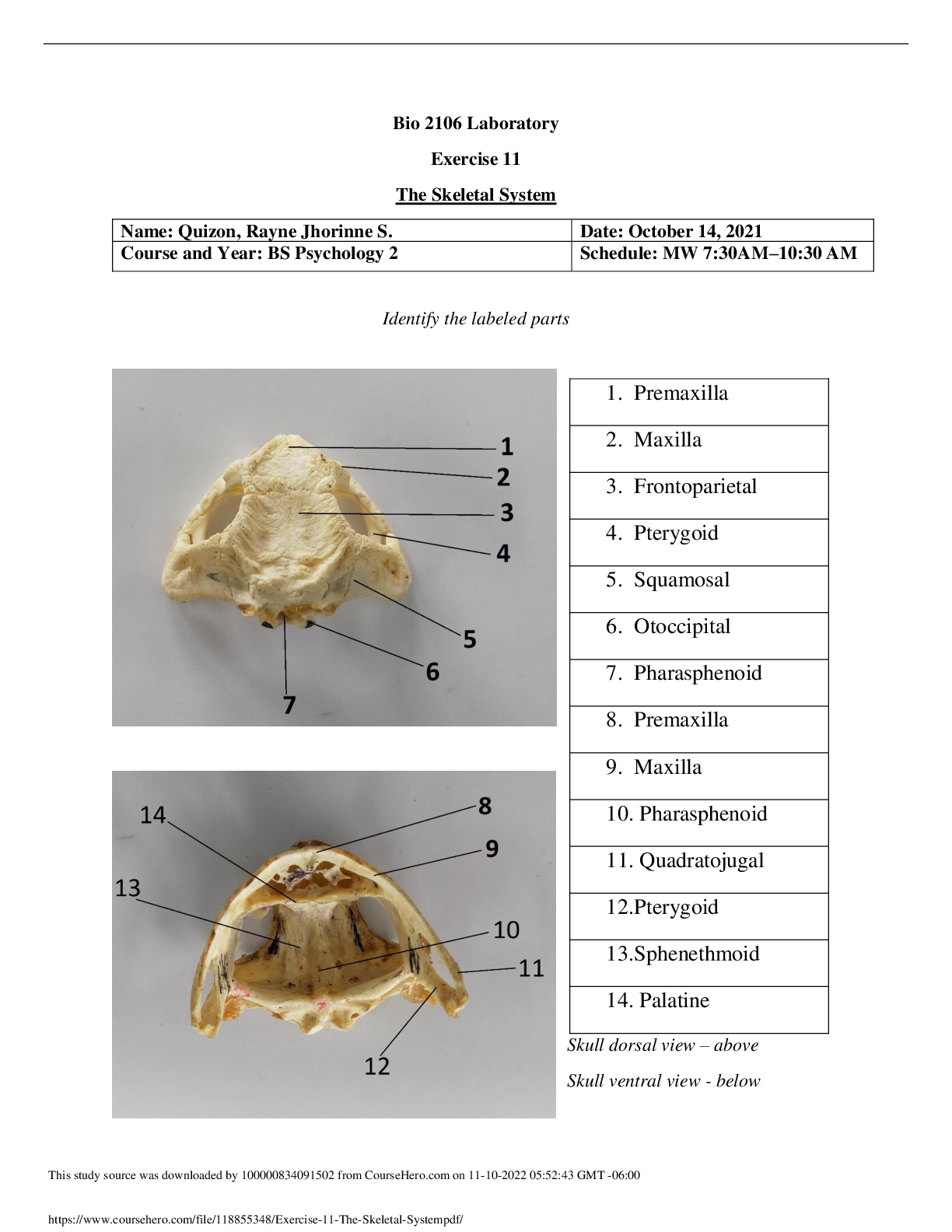
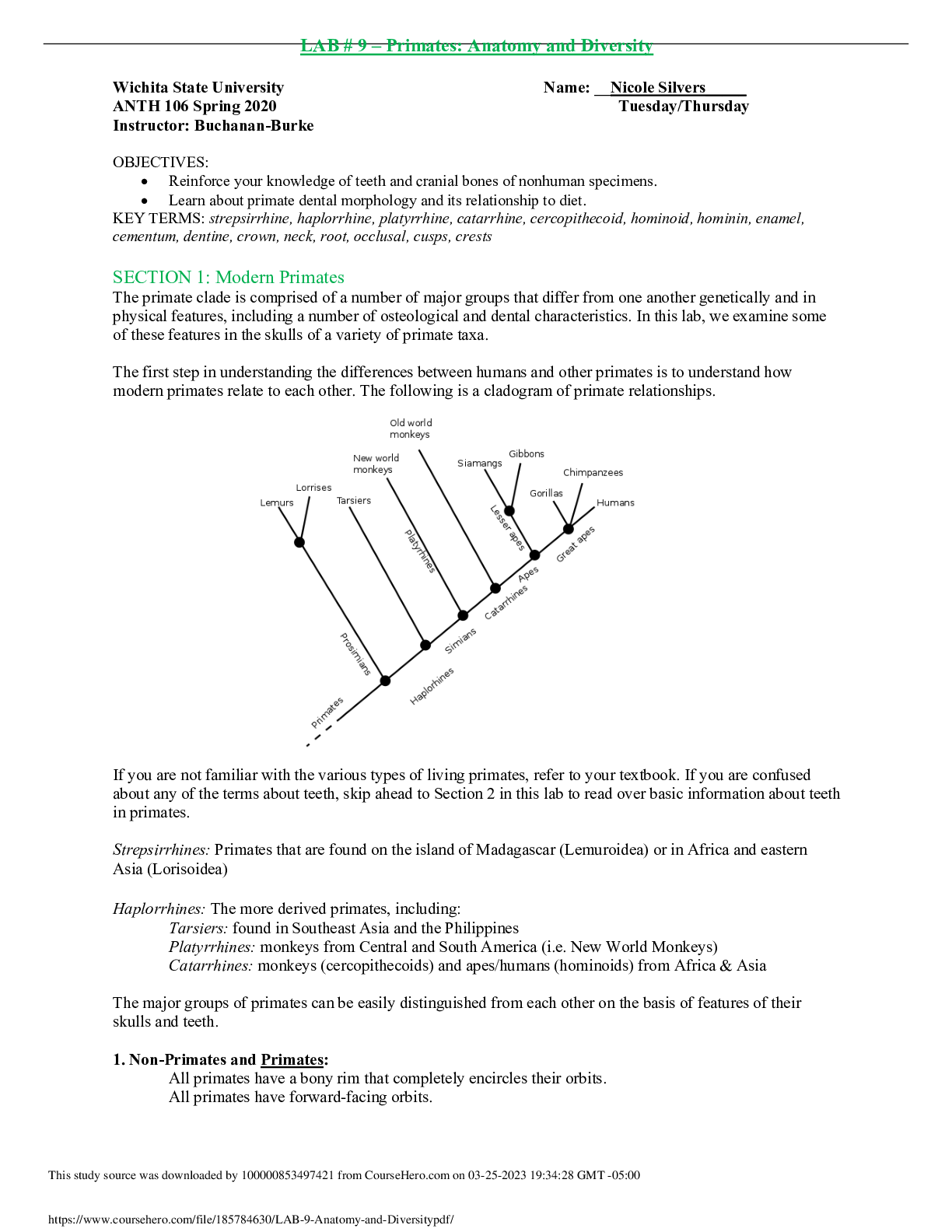
Isotope information: Total number of bean element atoms in cup = __22__ Isotope # of beans Mass of beans Average mass of one bean blackium 5 6 g 1.2 g brownium 8 11 g 1.375 g greenium 2 3.5 g 1.75... g whitium 7 9.6 g 1.37 g 1. What is an isotope? Isotopes are variants of a particular chemical element which differ in neutron number. 2. Explain any differences between the atomic mass of your BEANIUM sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used. Some differences were the mass of the beans, the % of beans, and the total number of beans. The difference would be smaller because the larger the sample the value of the masses would become closer. 3. What is the relationship between an element’s isotopes and the element’s atomic mass? The atomic mass of an element is equal to the sum of the protons and the neutrons in its nucleus. Isotopes are atoms of the same element that have a different mass number but the same atomic number 4. What is meant by a weighted average? The weighted average is the average atomic mass of a specific element. 5. How are the beans in this lab similar to isotopes? How are they different? They are similar because they have individual masses that are similar but not identical. They different becuase isotypes differ in mass but never atomic number. 6. What are at least two sources of error for this activity. (A source of error is an error that could have affected your results.) Two sources of error could be measuring mass wrong of the beans and not caculating the % of the beans correctly. 12.04 amu Determine the average atomic mass for the following mixtures of isotopes: 1.) Au – 197 = 50%, Au [Show More]
Last updated: 2 years ago
Preview 1 out of 3 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$3.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 09, 2022
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Nov 09, 2022
Downloads
0
Views
95