Chemistry > QUESTIONS & ANSWERS > CHEM101 Topic 9: Properties of Gases[ALL ANSWERS 100% CORRECT] (All)
CHEM101 Topic 9: Properties of Gases[ALL ANSWERS 100% CORRECT]
Document Content and Description Below
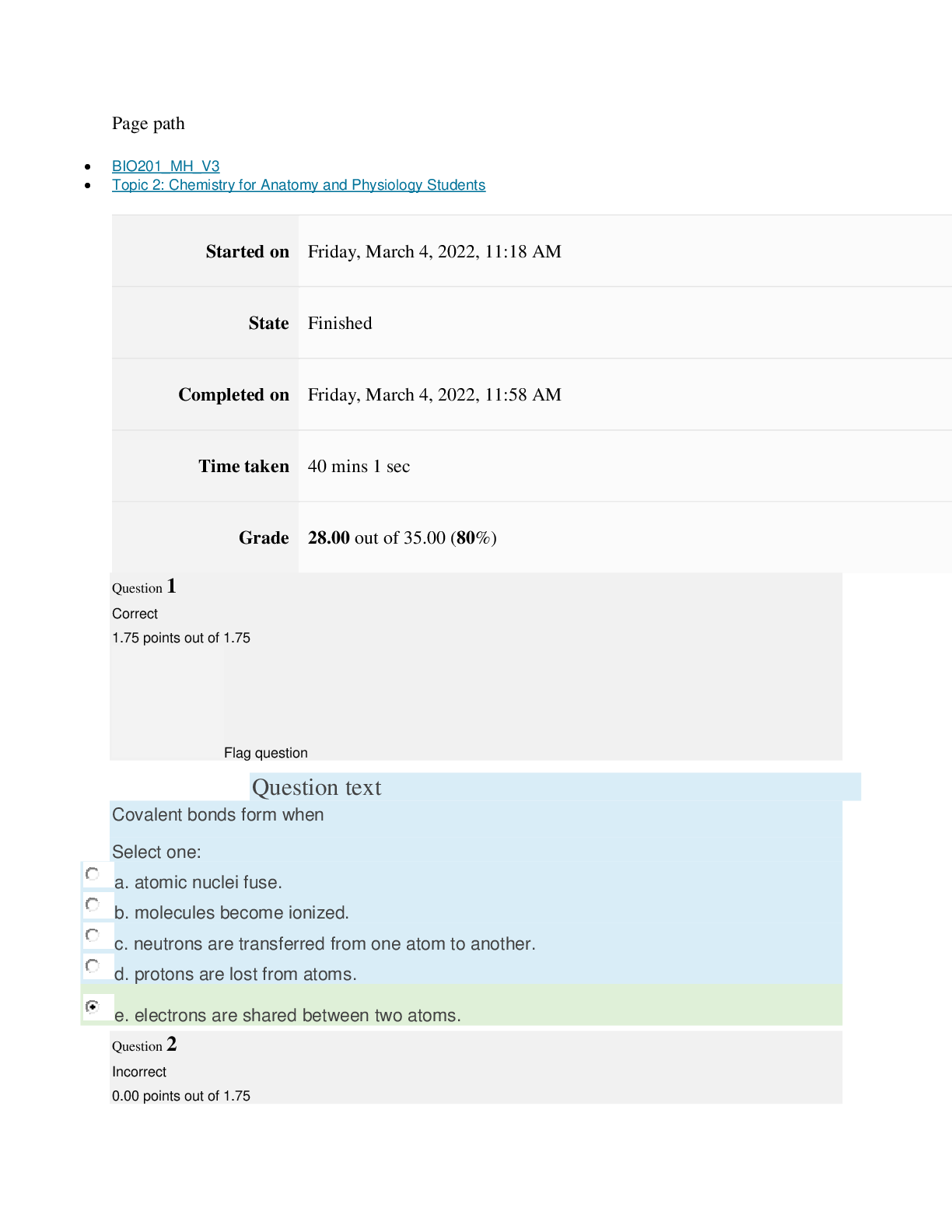
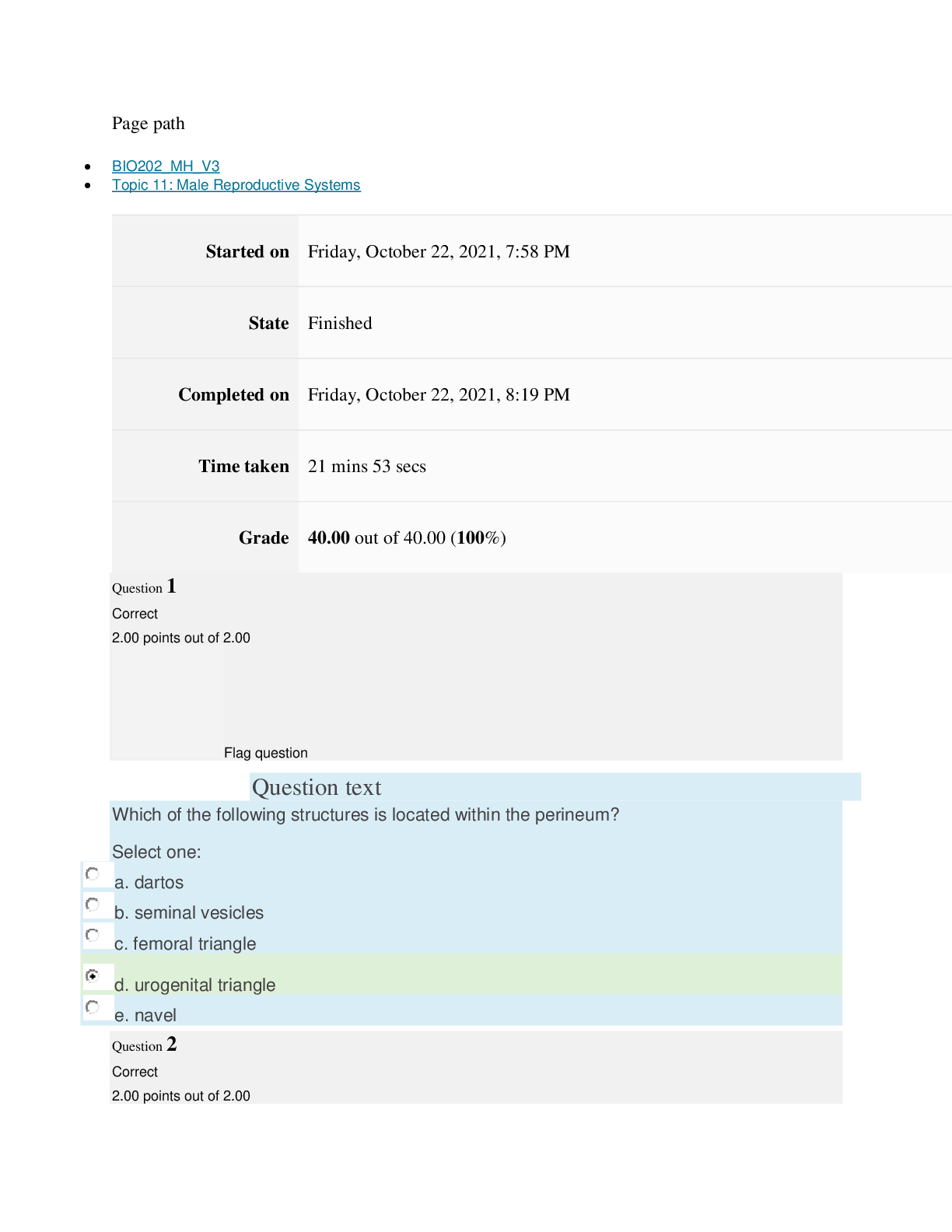
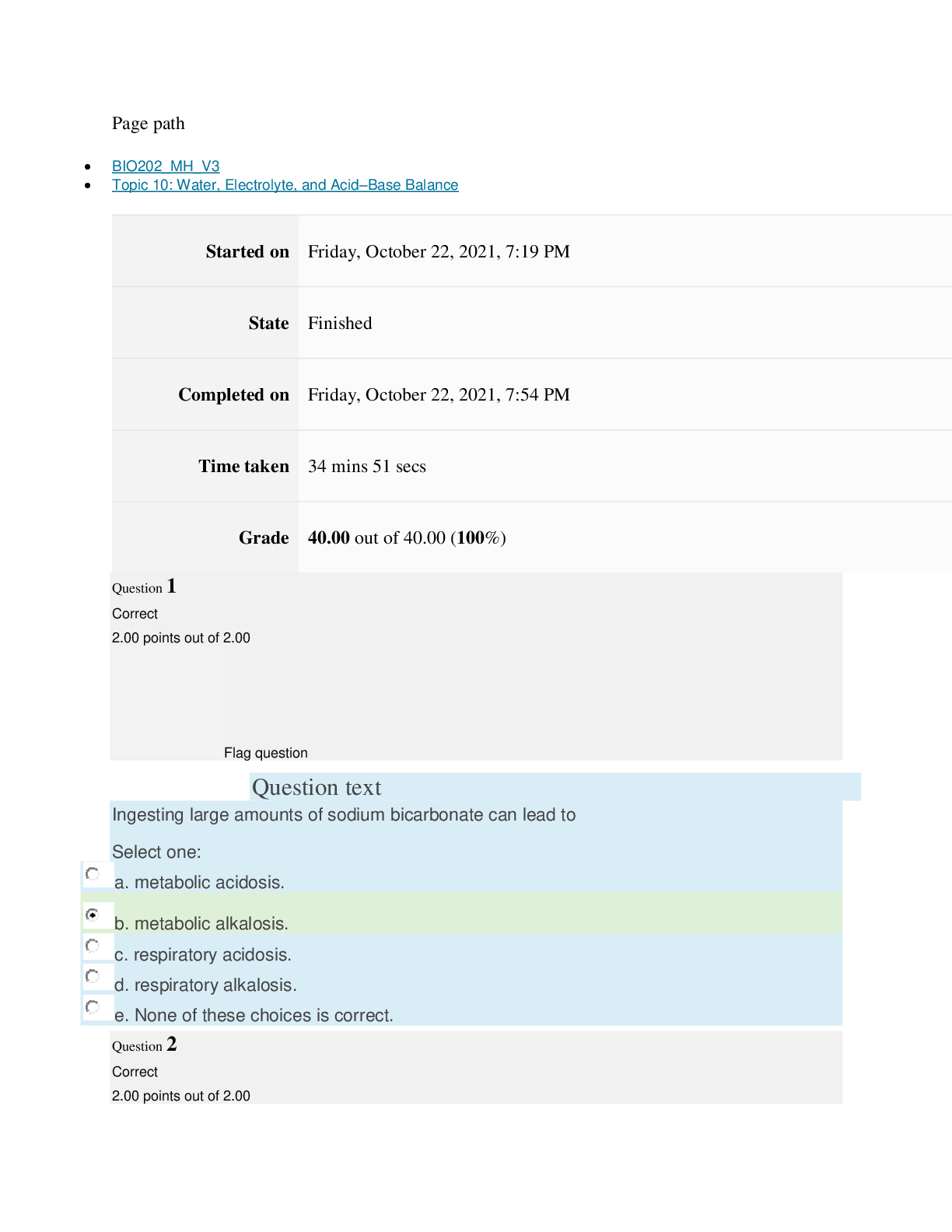
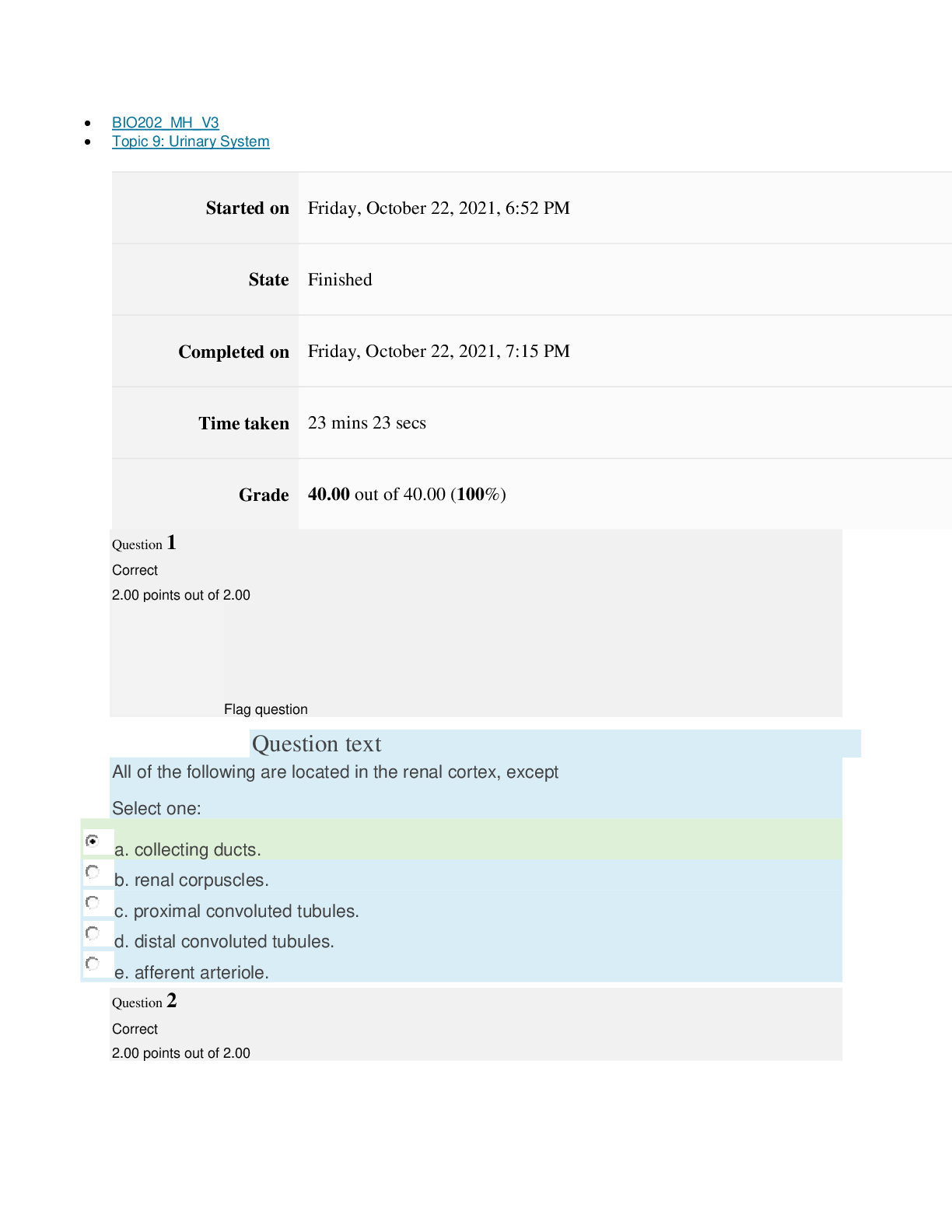
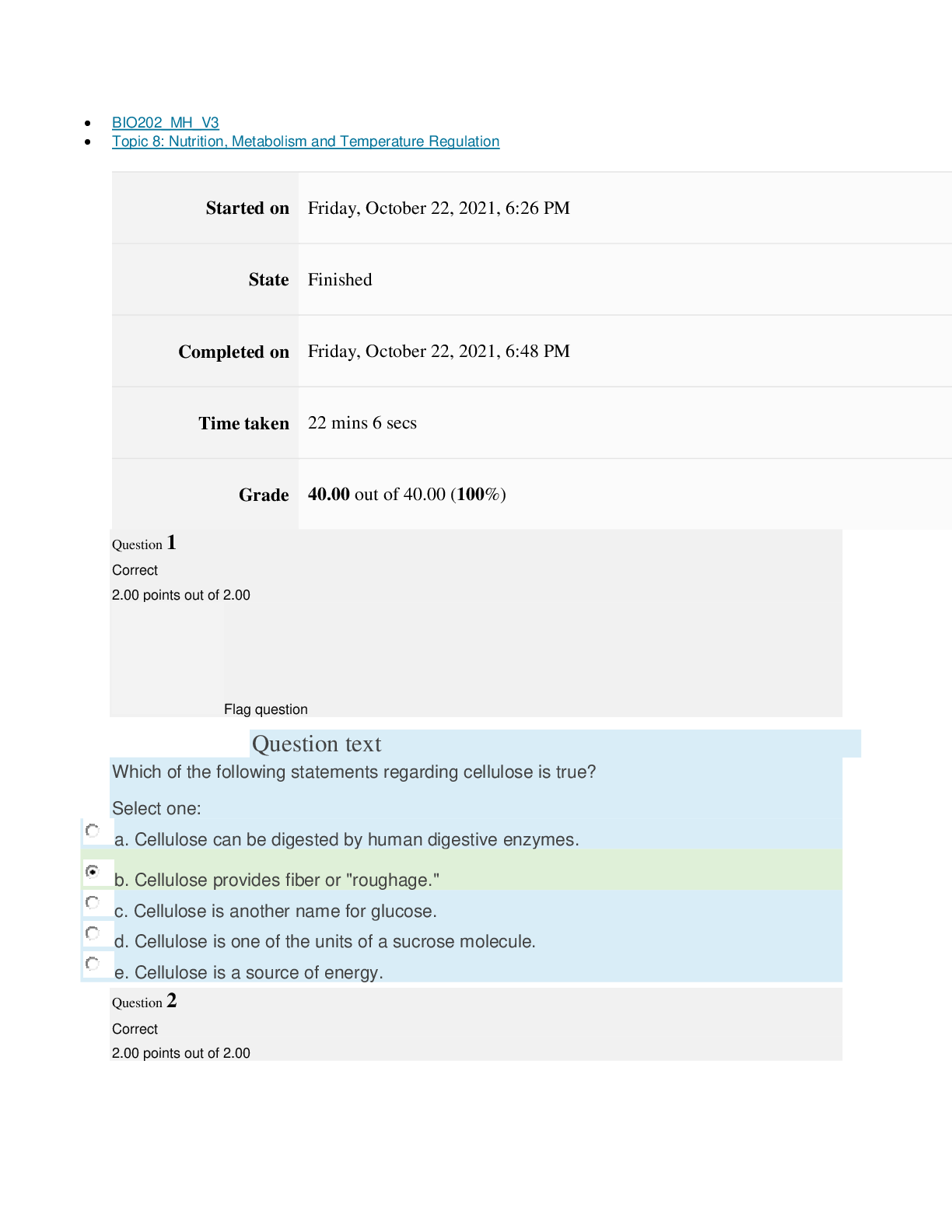
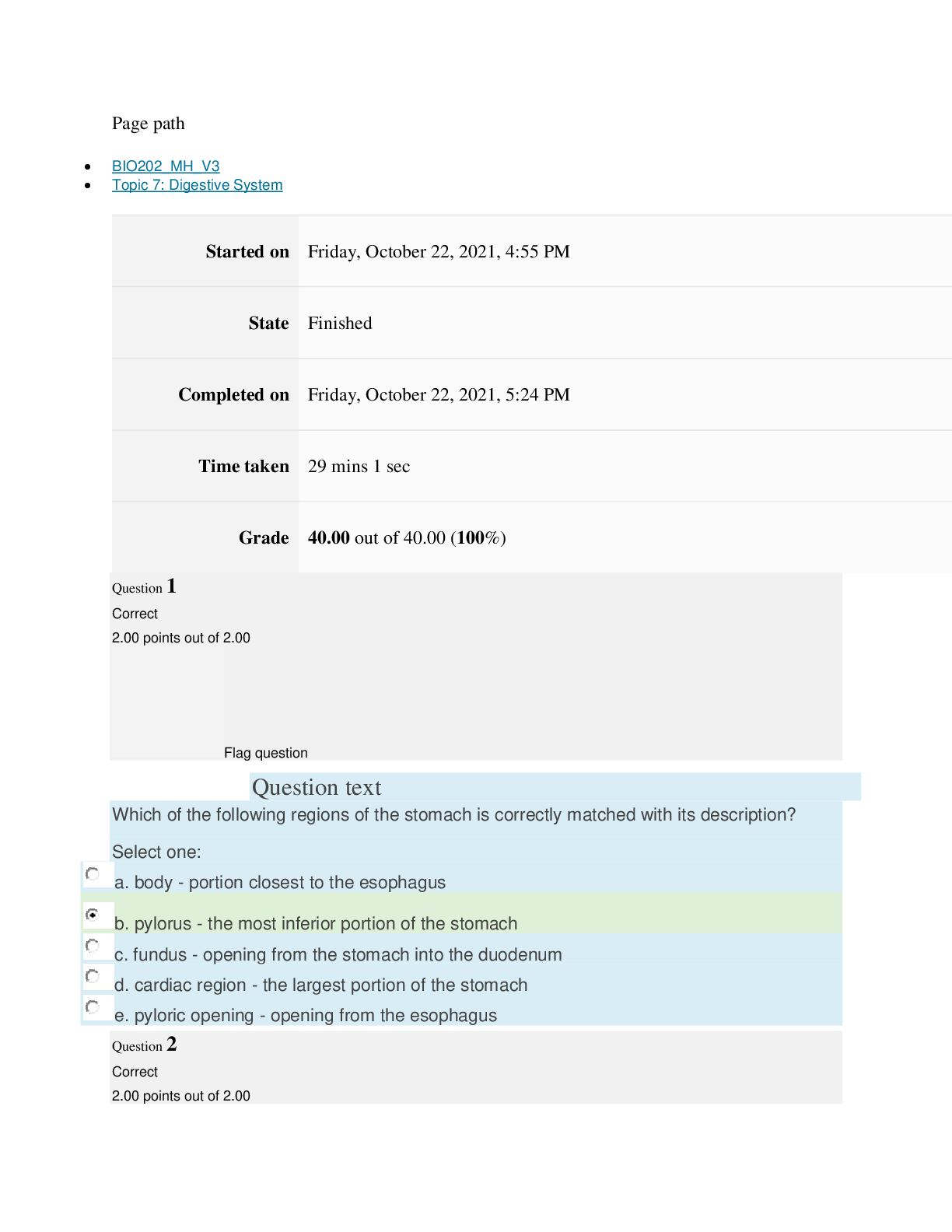
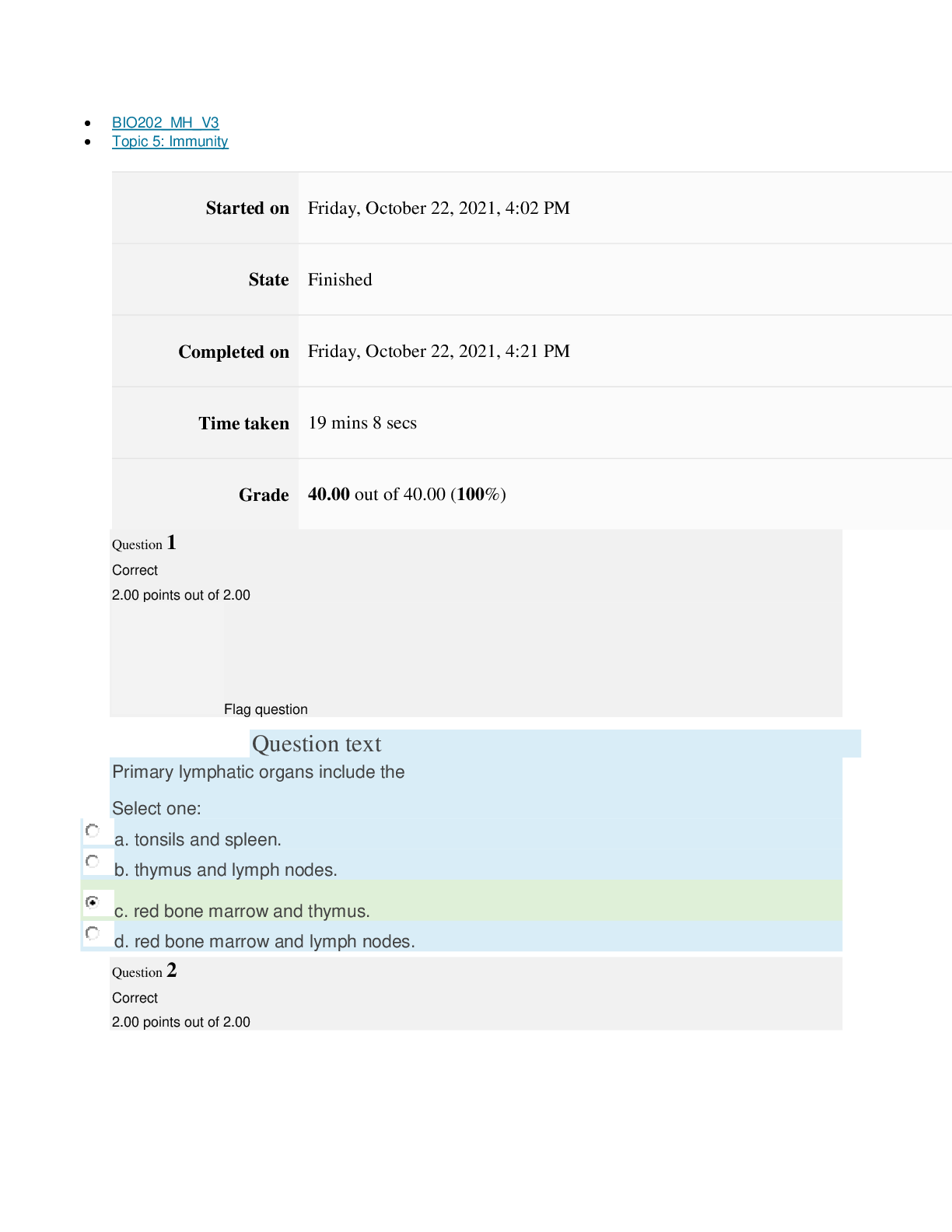
Page path CHEM101_MH_V4 Topic 9: Properties of Gases Started on Friday, April 15, 2022, 7:51 PM State Finished Completed on Friday, April 15, 2022, 8:09 PM Time taken 17 mins 47 secs Grade ... 115.00 out of 115.00 (100%) Question 1 Correct 4.60 points out of 4.60 Flag question Question text The nineteenth century chemists arranged elements in the periodic table according to increasing Select one: a. atomic number. b. number of electrons. c. atomic mass. Question text Which one of these ions is not isoelectronic with Kr? Select one: Question text Elements with first ionization energies and electron affinities generally form cations. d. high, very negative e. None of these is generally correct. c. low, positive or slightly negative Select one: a. low, very negative b. high, positive or slightly negative Flag question Question 3 Correct 4.60 points out of 4.60 b. Se2– c. Rb+ d. Sr2+ e. Br– a. As3+ d. number of neutrons. e. nuclear binding energy. Flag question Question 2 Correct 4.60 points out of 4.60 Question 4 Correct 4.60 points out of 4.60 Flag question Question text Which ground-state ion does not have an electron configuration described by the following orbital diagram? Select one: Question text An element with the electron configuration [noble gas]ns2(n – 1)d8 has valence electrons. e. None of these choices is correct. [Show More]
Last updated: 2 years ago
Preview 1 out of 13 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
13
Written in
Additional information
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
96