
NRNP 6635 MIDTERM 2023 Latest Questions and Answers All CorrectStudy Guide
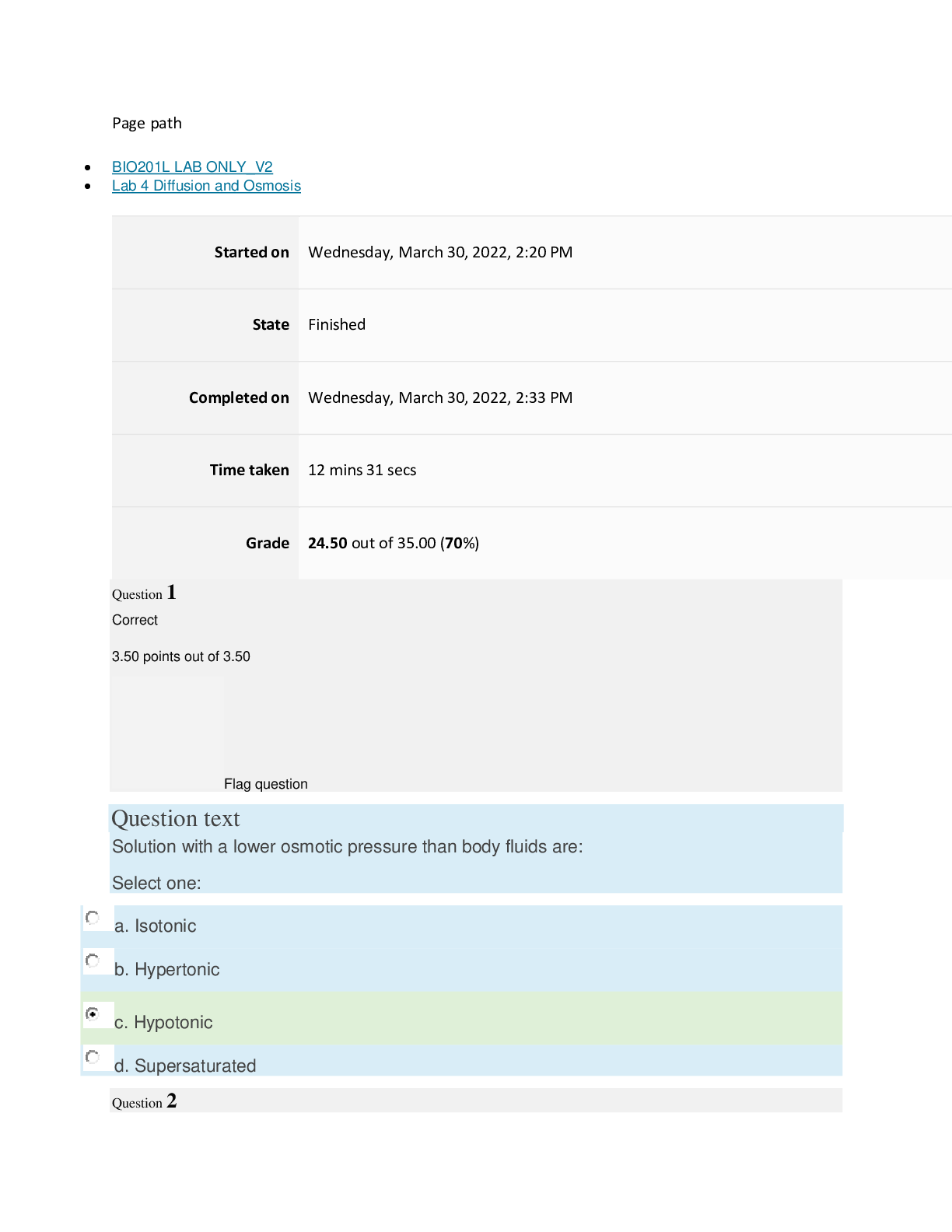
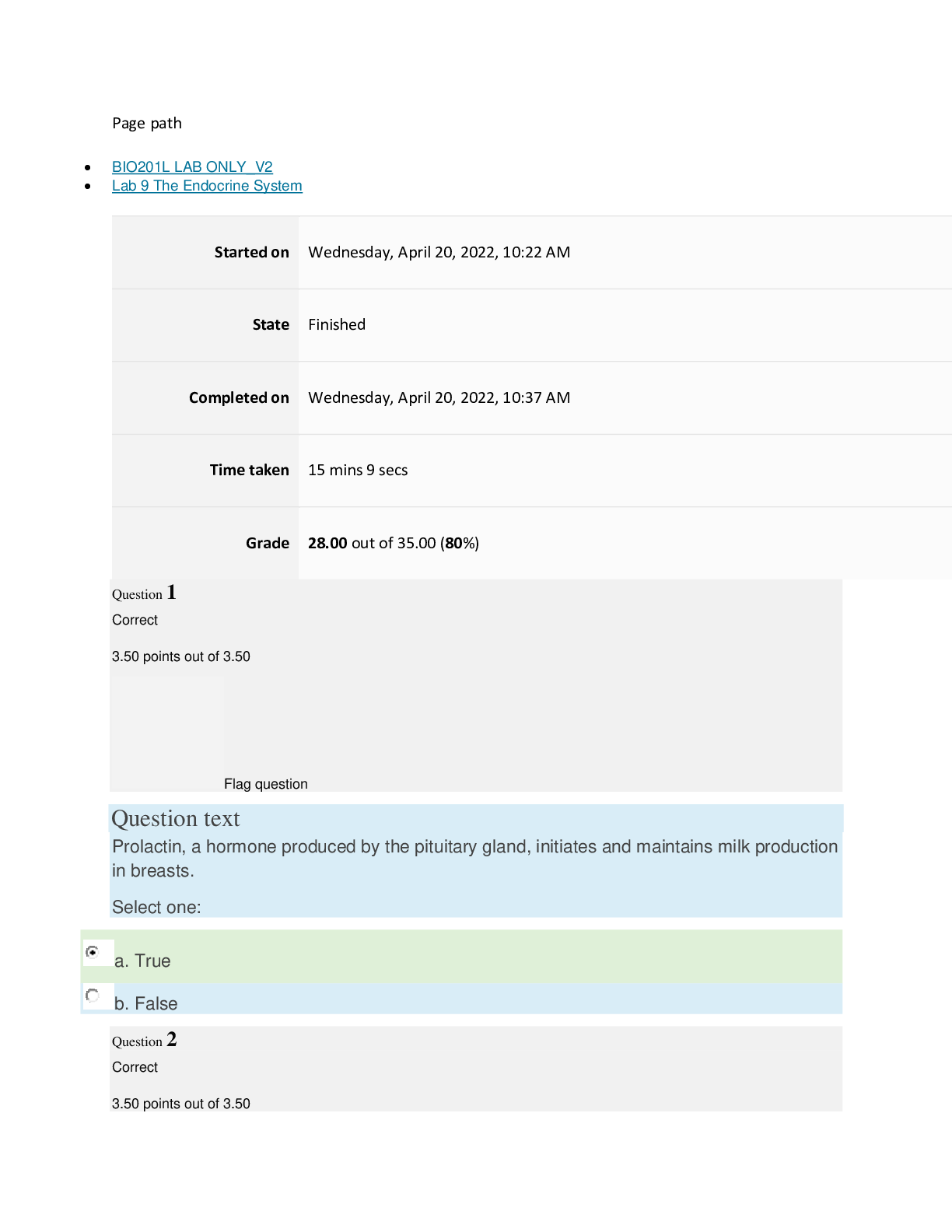
Page path BIO201L LAB ONLY_V2 Lab 4 Diffusion and Osmosis Started on Wednesday, March 30, 2022, 2:20 PM State Finished Completed on Wednesday, March 30, 2022, 2:33 PM Time taken 12 mins ... 31 secs Grade 24.50 out of 35.00 (70%) Question 1 Correct 3.50 points out of 3.50 Flag question Question text Solution with a lower osmotic pressure than body fluids are: Select one: a. Isotonic b. Hypertonic c. Hypotonic d. Supersaturated Question 2 Correct 3.50 points out of 3.50 Flag question Question text Identify the units used to define molarity. Select one: a. Grams/Liter b. Moles/Liter c. Grams/Mole d. Liters/Mole Question 3 Correct 3.50 points out of 3.50 Flag question Question text Osmotic pressure is directly correlated with tonicity. Select one: a. True b. False Question 4 Incorrect 0.00 points out of 3.50 Flag question Question text Which of the following describes the relationship between osmotic pressure and water potential? Select one: a. High Osmotic Pressure = High Water Potential b. High Osmotic Pressure = Low Water Potential c. Low Osmotic Pressure = Low Water Potential d. None of the above. Question 5 Incorrect 0.00 points out of 3.50 Flag question Question text Osmotic pressure is the force required for osmosis to occur. Select one: a. True b. False Question 6 Incorrect 0.00 points out of 3.50 Flag question Question text What is the main aquaporin that is found in the body? Select one: a. Aquaporin 1 b. Aquaporin 2 c. Aquaporin 3 d. Aquaporin 4 Question 7 Correct 3.50 points out of 3.50 Flag question Question text An equitonic solution has equal solute concentrations on both sides of the permeable membrane. Select one: a. True b. False Question 8 Correct 3.50 points out of 3.50 Flag question Question text Diffusion and osmosis are examples of which type of transport? Select one: a. Regulated b. Mechanical c. Passive d. Active Question 9 Correct 3.50 points out of 3.50 Flag question Question text What is the difference between diffusion and osmosis? Select one: a. Osmosis states the molecules move from high to low concentration, whereas diffusion states that molecules move from low to high concentration. b. Diffusion does not consider semi-permeable membranes, whereas osmosis only considers semi-permeable membranes. c. Diffusion refers only to water molecules, while osmosis refers to any molecule. d. Osmosis refers to water molecules, while diffusion refers to any molecule. Question 10 Correct 3.50 points out of 3.50 Flag question Question text In a solution, substances are dissolved in a: Select one: a. Solvent b. Solute c. Mixture d. None of the above [Show More]
Last updated: 3 years ago
Preview 1 out of 6 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

BIO201L: LAB QUIZES
By Book Worm, Certified 3 years ago
$24
11
Can't find what you want? Try our AI powered Search
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
6
Written in
All
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
195
Scholarfriends.com Online Platform by Browsegrades Inc. 651N South Broad St, Middletown DE. United States.
We're available through e-mail, Twitter, Facebook, and live chat.
FAQ
Questions? Leave a message!
Copyright © Scholarfriends · High quality services·