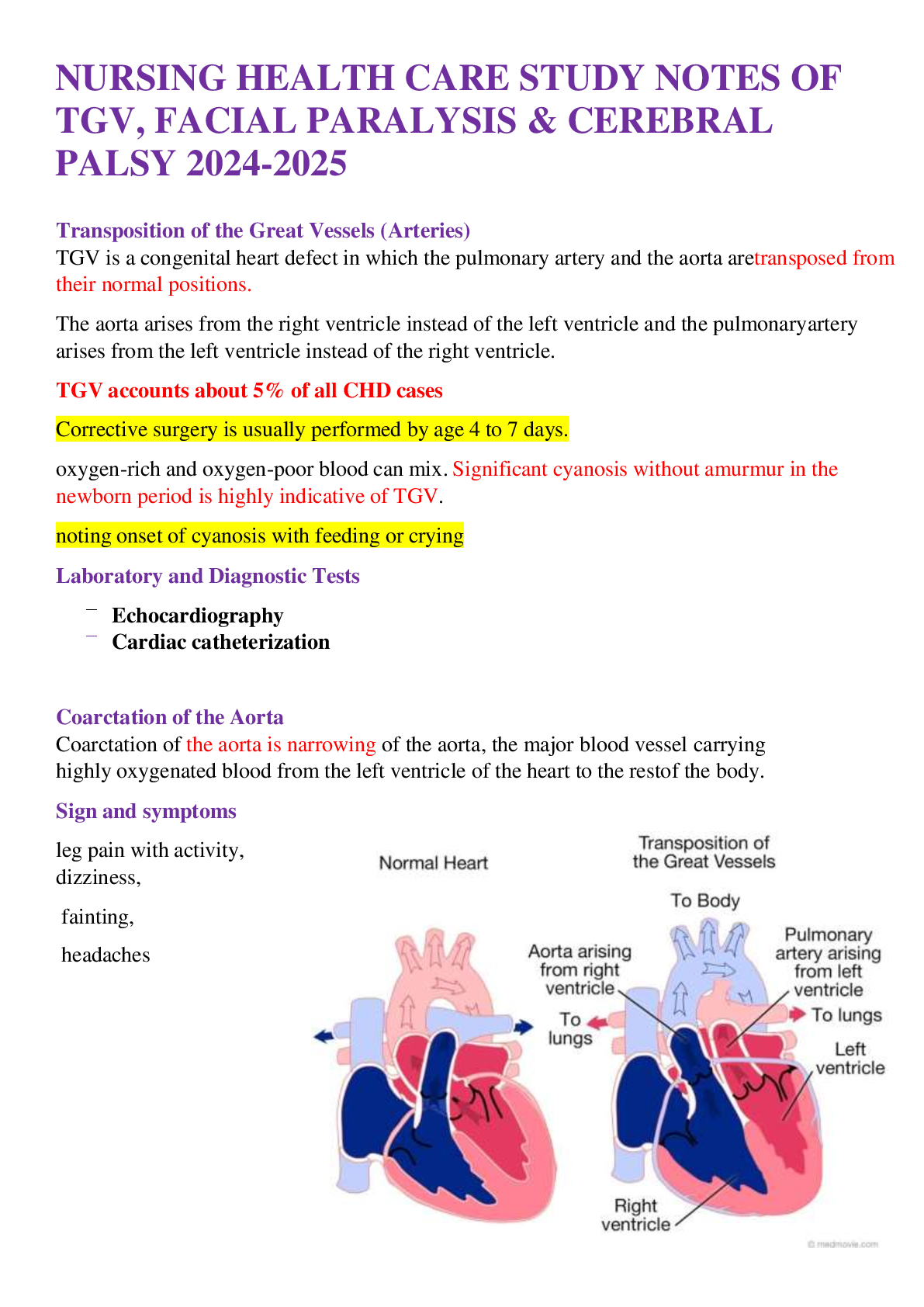
The Federal Regulations - SBE
Content Author
Lorna Hicks, MS
Duke University
Introduction
Revelations in the early 1970s about egregious medical experiments provided the imp
...
The Federal Regulations - SBE
Content Author
Lorna Hicks, MS
Duke University
Introduction
Revelations in the early 1970s about egregious medical experiments provided the impetus for developing federal standards for protecting human research subjects;
however, a close reading of the regulations at 45 CFR 46 will find mention of research methods and topics of inquiry relevant to researchers in the social and behavioral sciences, as well as education and the humanities. Methods mentioned include surveys, interviews, focus groups, participant observation, observations of public behavior, and the analysis of existing data. Topics include research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social
behavior.
In addition to explicitly identifying these methods, the regulations include provisions that allow for appropriate review of social sciences, humanities, and behavioral
research. For example, the regulations:
Identify research activities that are low risk (for example, surveys in which no identifiers are collected), and that are exempt from some provisions of the
regulations (such as the requirement for continuing review).
Identify research activities with no more than minimal risk that can be initially
reviewed by one or more Institutional Review Board (IRB) members, rather than at a convened IRB meeting.
Allow for waivers of the requirement to obtain written consent (for example, in a study of undocumented workers).
Include provisions that permit researchers to withhold information in the consent process. This provision is important when some degree of deception is required in order to obtain valid results.
Allow for the amendment of approved study plans. This process can be used
effectively when it is not possible to know at the outset how a study will evolve. An example would be field research.
This module provides an overview of the federal regulations, so researchers can become familiar with the basic provisions. The full text of the federal regulations is available
online.
Learning Objectives
By the end of this module, you should be able to:
Determine whether proposed research meets the criteria for exemption.
Describe the criteria for the use of expedited review procedures and IRB review.
Summarize the authority of an IRB.
Describe the kinds of review that approved research may need.
45 CFR 46: Protection of Human Subjects
[Show More]