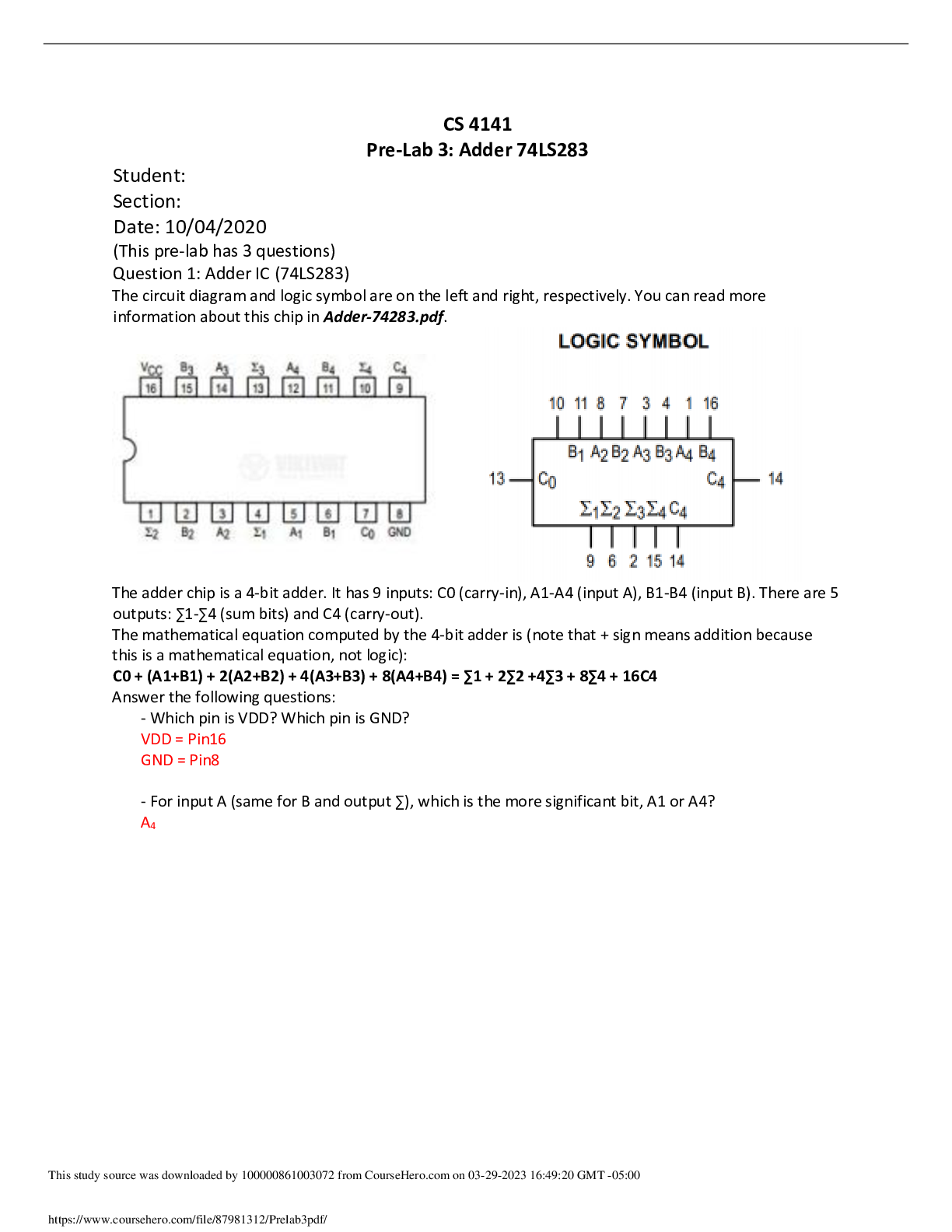
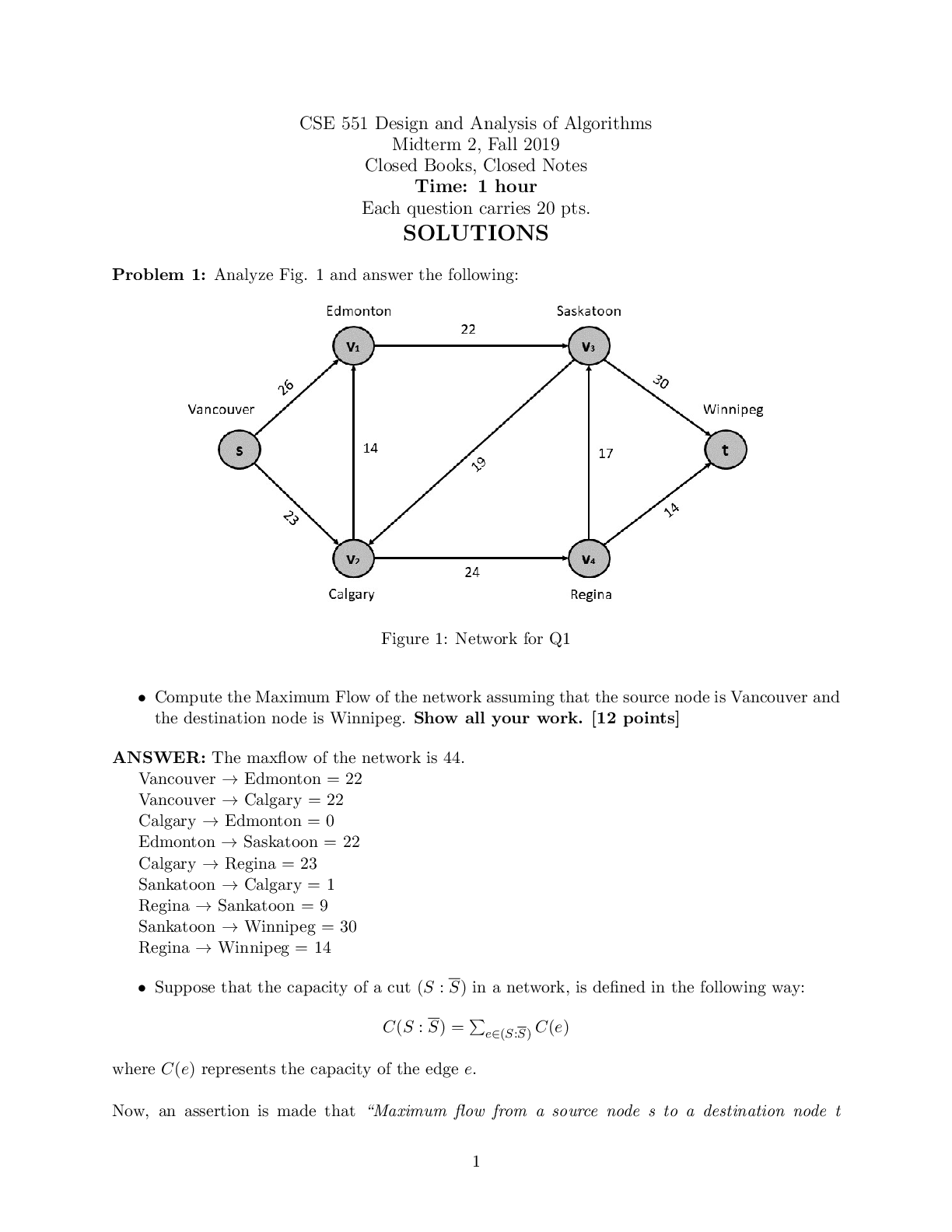
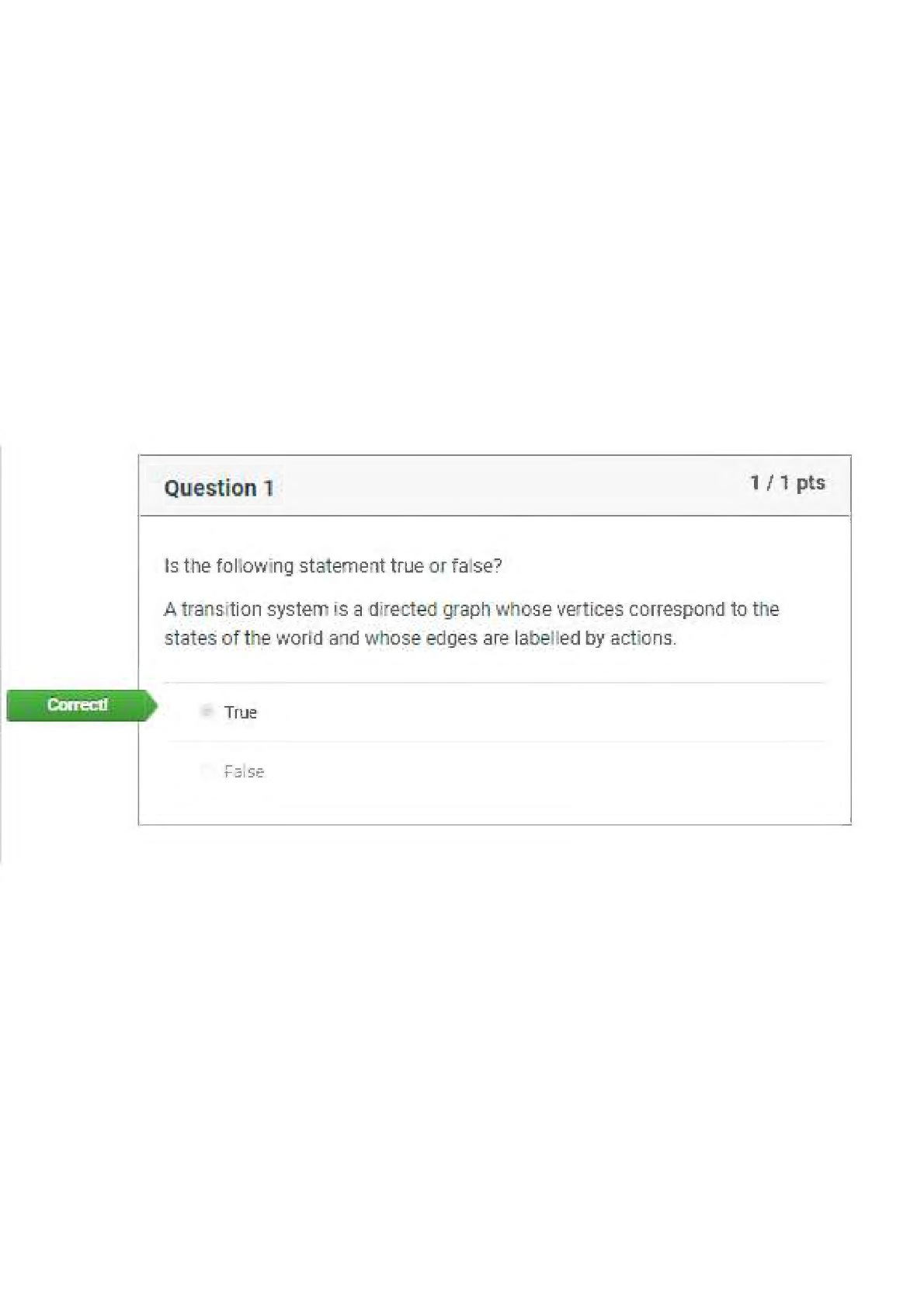
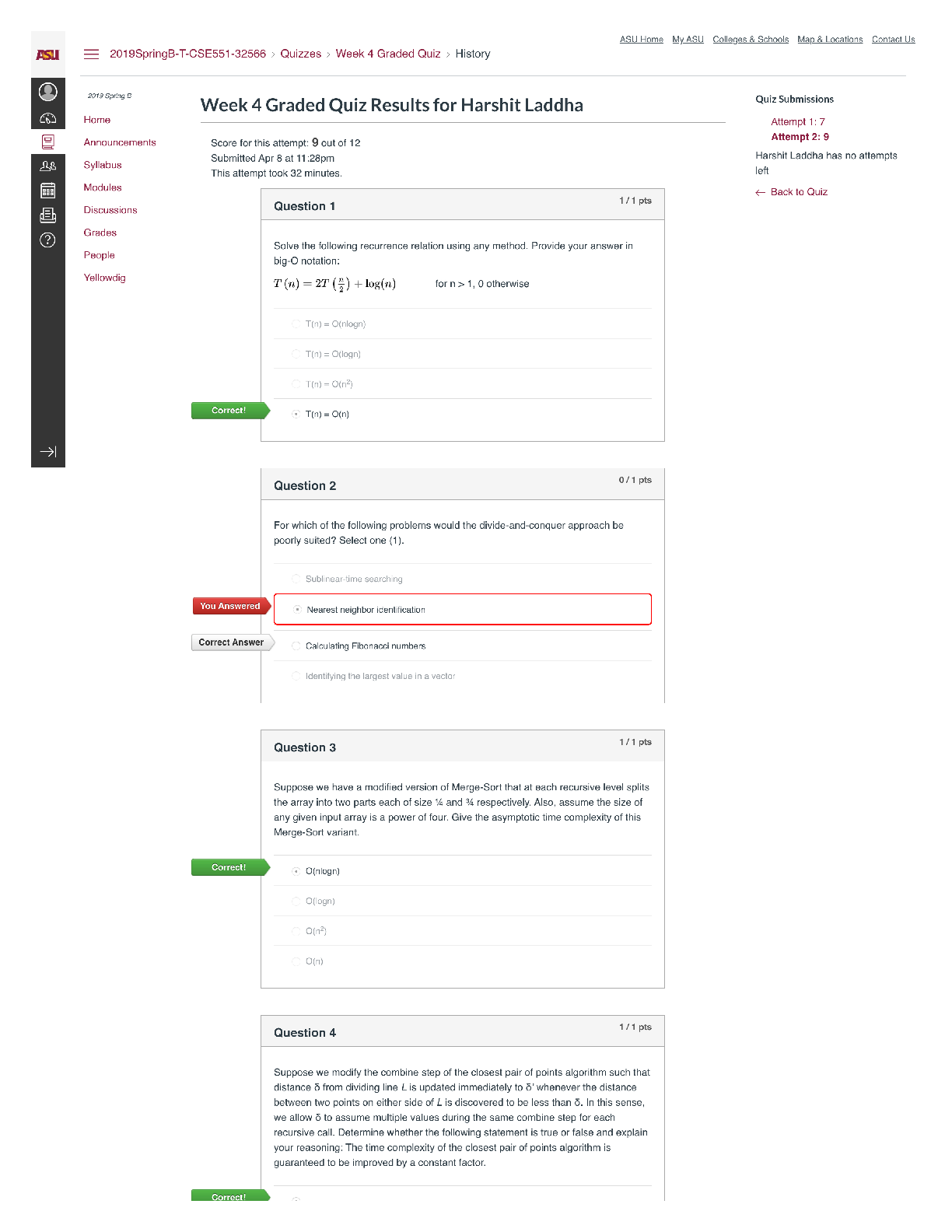
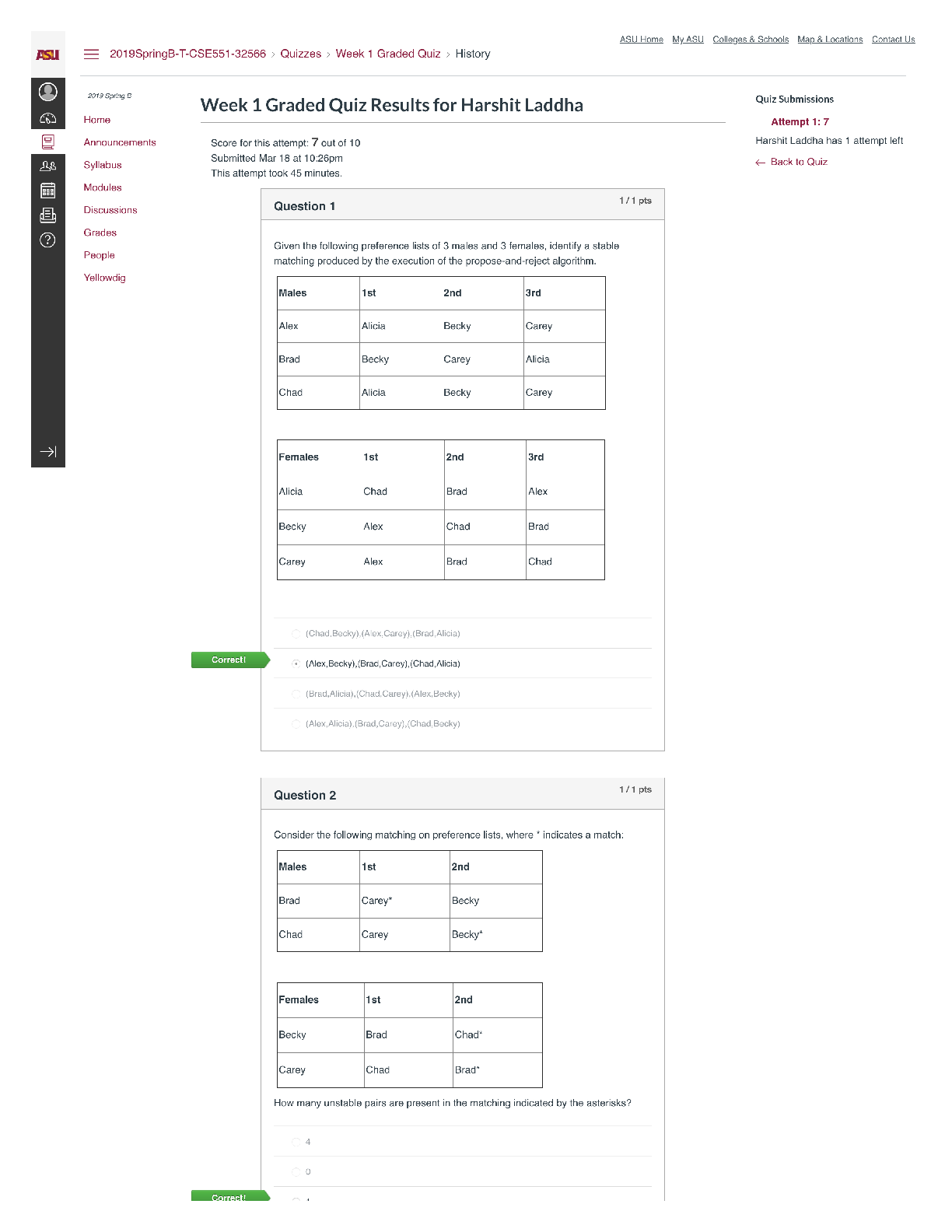
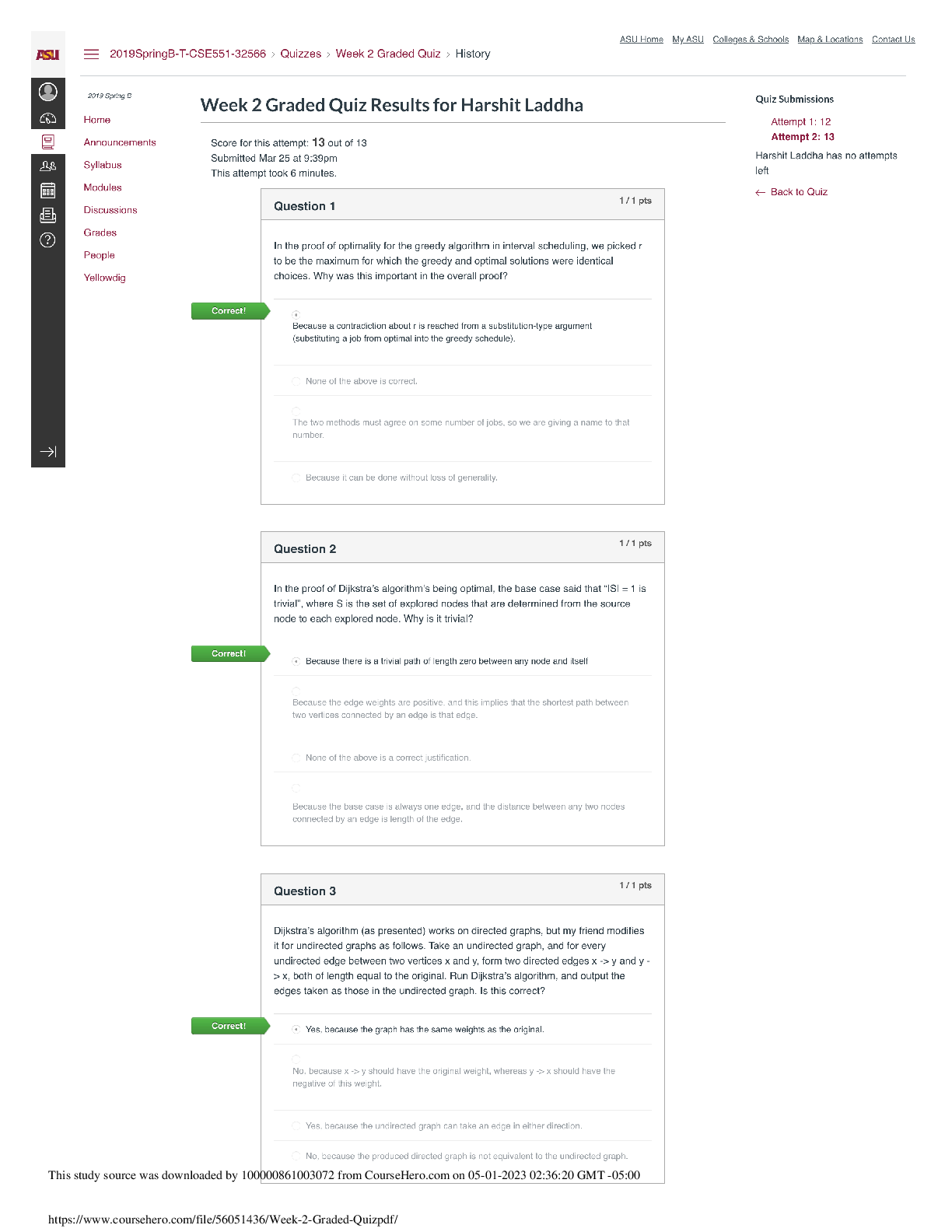
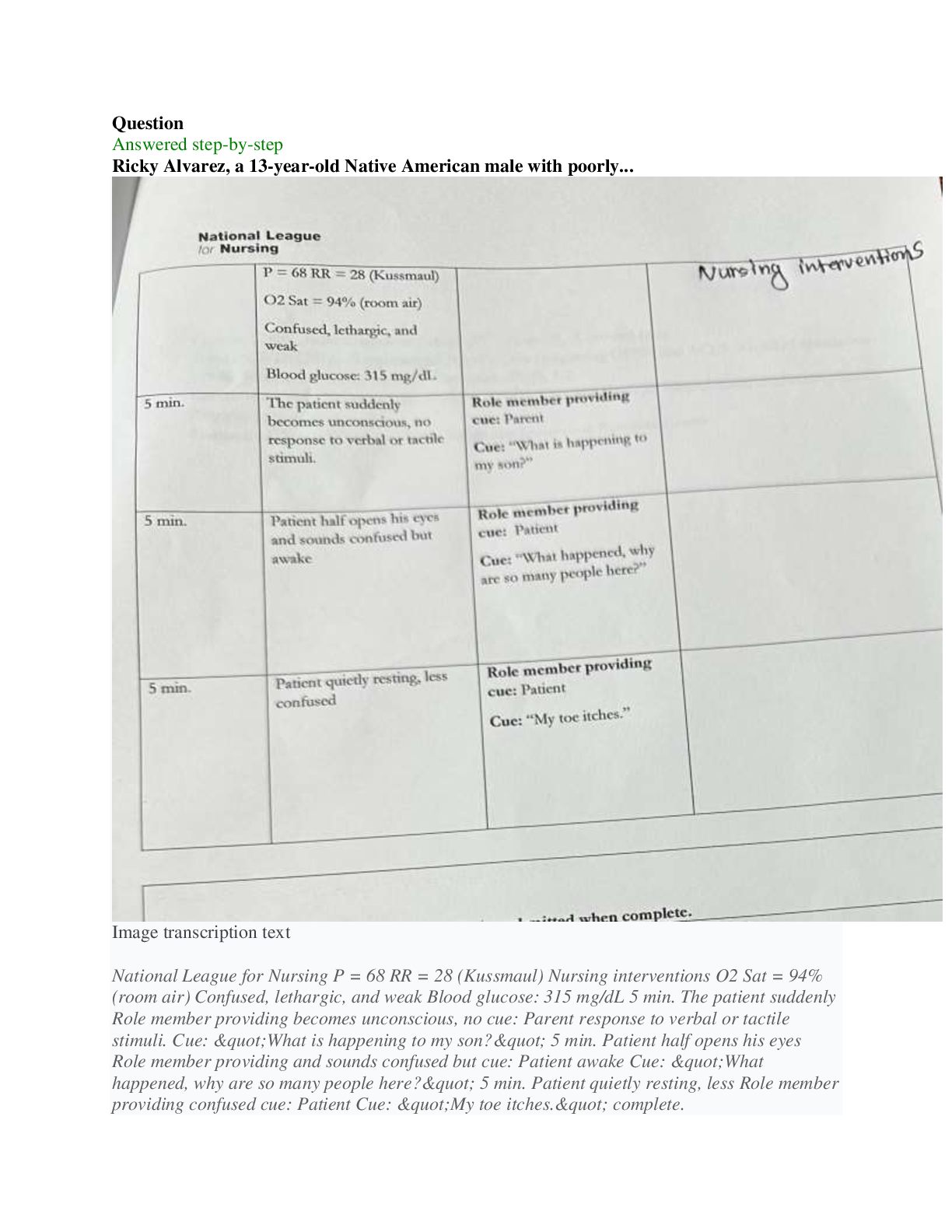
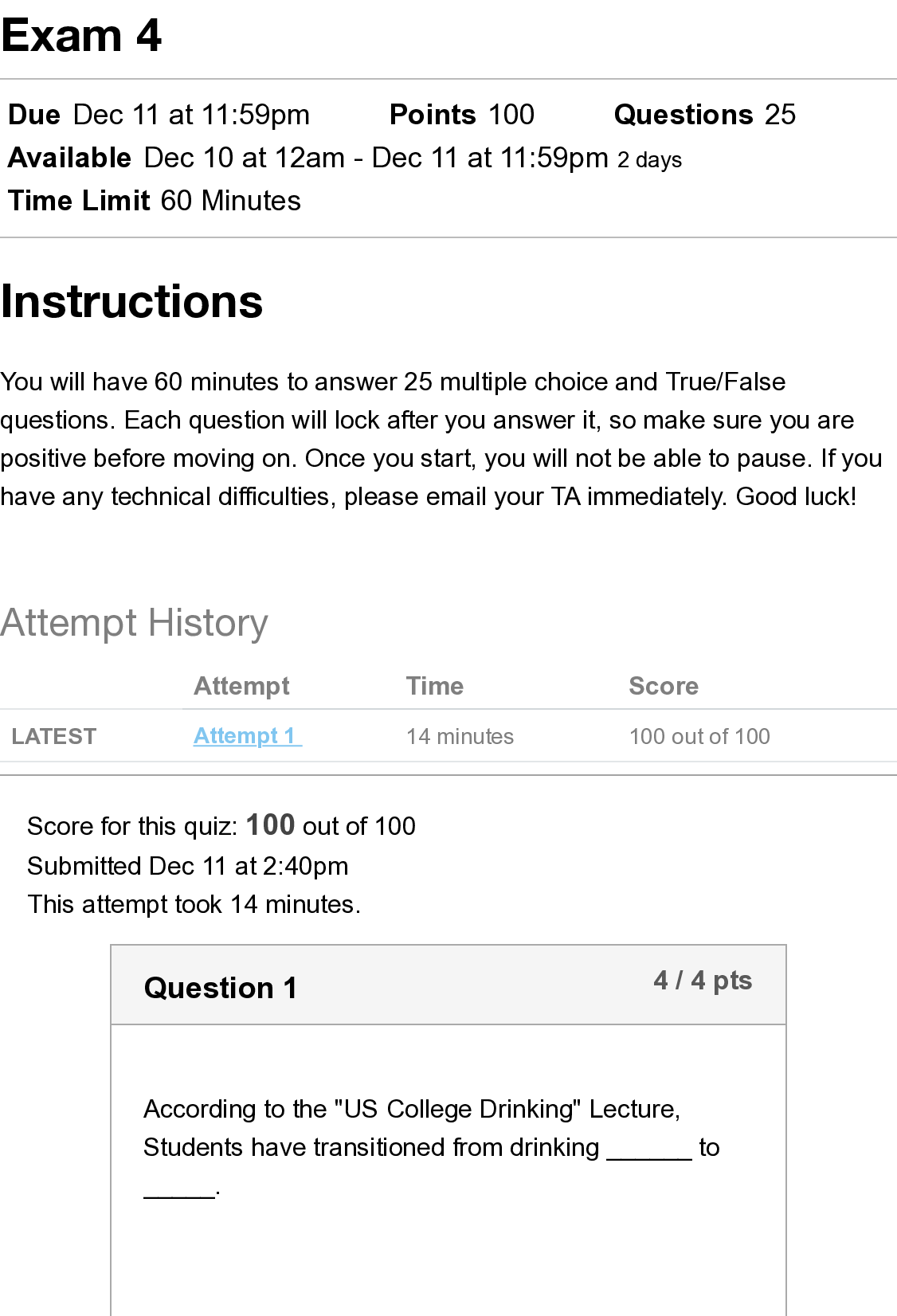
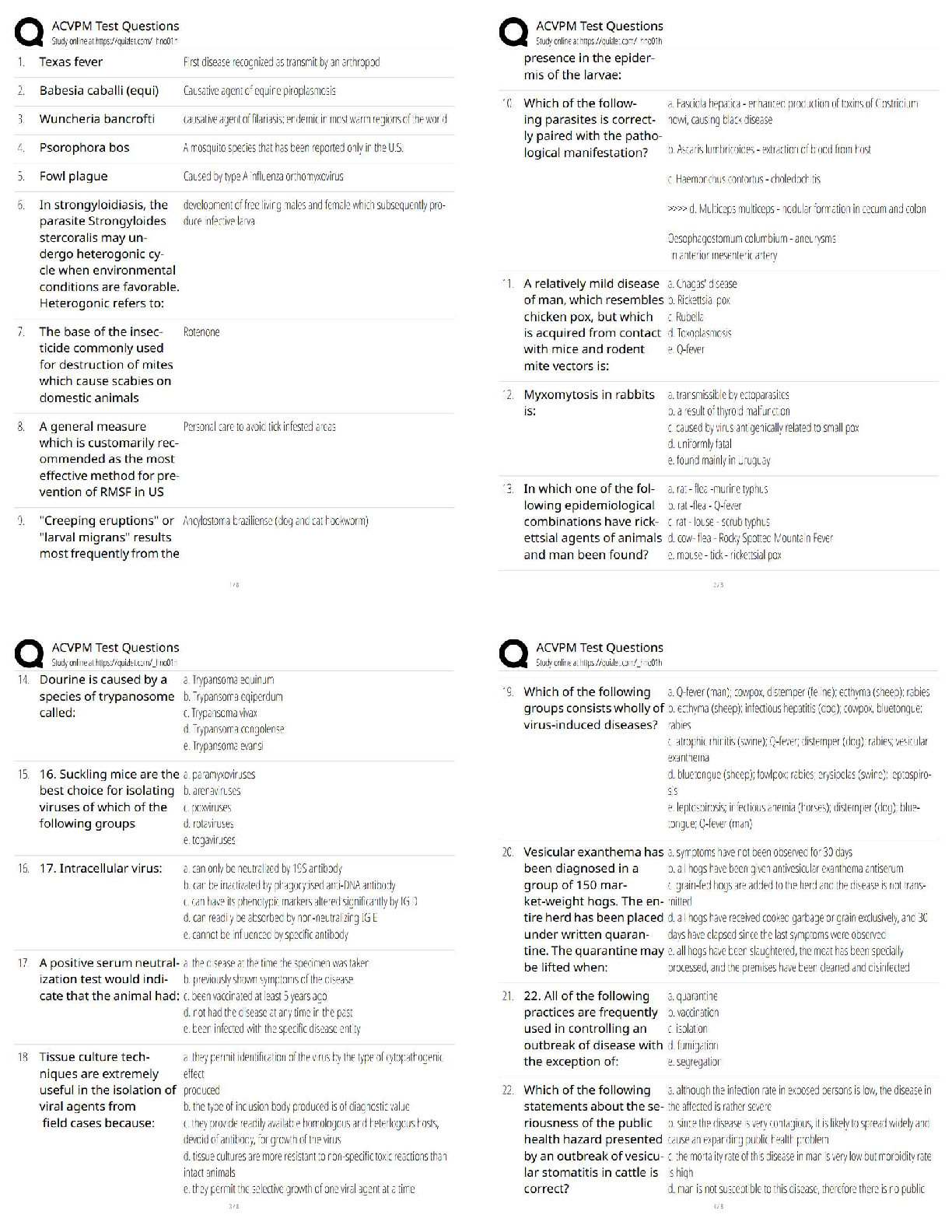
BIOS 255 Week 5 Virtual Lab: Introduction to Immunology Part I, Part II
$ 10

Chem 120) Chemistry Final Review:
$ 8.5

Exit HESI Test Bank (over 1000 Q's and Answers ) spring 2023 / Exit HESI Prep Distinction Level Assignment Has everything A+
$ 9.5

TEST BANK FOR UNDERSTANDING ANATOMY & PHYSIOLOGY: A VISUAL, AUDITORY, INTERACTIVE APPROACH (4TH ED.) BY GALE SLOAN THOMPSON |ALL CHAPTERS | ANSWERS WITH RATIONALES NEWEST VERSION
$ 7.5

NC Drivers Ed Final Exam Practice Test With Complete Updated Solutions 2023-2024
$ 18

BIOCHEM C785 Kaleys Comprehensive Study Guide final (Completed ) BIOCHEM C785 Kaleys Comprehensive Study Guide final
$ 18.5

Pearson Edexcel_GCSE (9-1) History_1HI0/11 Mark Scheme 2020 | Paper 1: Thematic study and historic environment
$ 6.5

[eBook] [PDF] Tomorrow's Data Empowered Project Management By Öncü Hazır, Maria Elena Bruni
$ 30
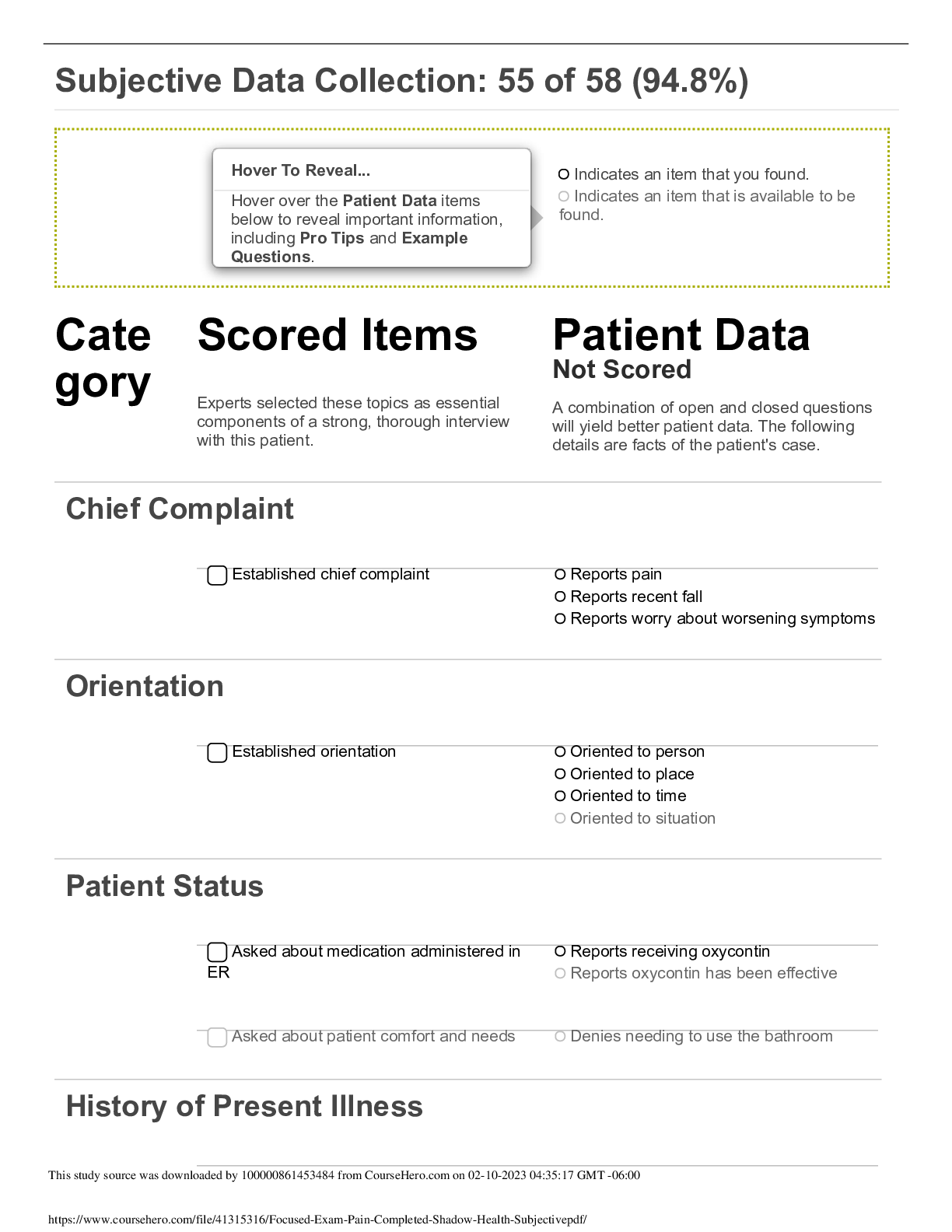
Focused Exam: Pain Completed Shadow Health-QSEN Subjective Data /LATEST 2022/2023 /100% Verified
$ 10

POSTEST ATLS QUESTIONS And ANSWERS 2023 Update
$ 11

Operator Theory A Comprehensive Course in Analysis, Part 4 Barry Simon
$ 12

Law M100 Arbitration Exam Notes
$ 12

CHEM 120 Week 8 Final Exam LATEST UPDATE
$ 19

[eBook] [PDF] Construction Project Organising By Simon Addyman, Hedley Smyth
$ 25

(NURS 6053 Module 5 Assignment) / NURS 6053 Module 5 Assignment: Change Implementation and Management Plan (complete presentation)
$ 16

PORTAGE LEARNING BIOD 151 ALL LAB EXAM QUESTIONS AND 100% CORRECT ANSWERS LATEST UPDATE 2024
$ 18

Solution Manual for Cyberlaw The Law of the Internet and Information Technology 1st Edition by Brian
$ 17

Biochemistry C 785 Readiness Check 2020 – Western Governors University | Biochemistry C785 Readiness Check {A Grade}
$ 17

A&P 1 Module 1- 10 Quizzes + Module 11 Final Questions & Answers
$ 20
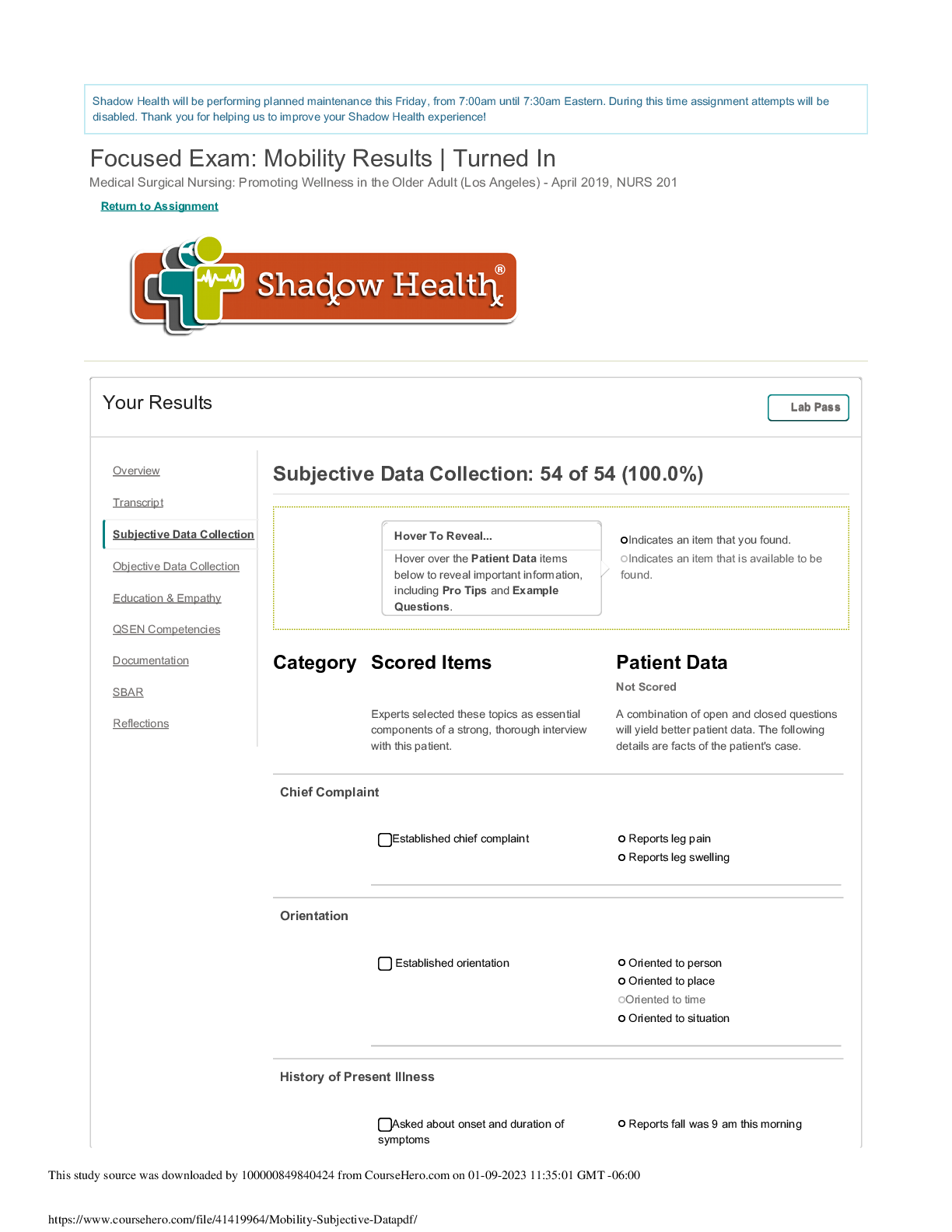
Mobility Subjective Data. Medical Surgical Nursing: Promoting Wellness in the Older Adult (Los Angeles), NURS 201
$ 13
.png)
Biochemistry REVISION QUESTIONS
$ 6

ATPL - AIRLAW 2/4 (PCAR TABLE) - Questions and Answers
$ 12
.png)
Liberty University - EDSP 370 Michael Jones IEP Part 1 & 2 Complete
$ 8

Financial Markets and Institutions, 10th edition Frederic S Mishkin test bank
$ 31.5

Student Exploration: Big Bang Theory – Hubble’s Law. GIZMO
$ 7

Chamberlain College of Nursing - CHEM 120/CHEM 120 Week # 7 DQ 1 & 2_Verified Answers.
$ 10

week 1 flashcards.docx The science in which tactics derived from the principles of beha
$ 5

RASMUSSEN COLLEGE ANATOMY BSC 2346 MODULE 7 QUIZ ANSWER,GRADED A.
$ 14
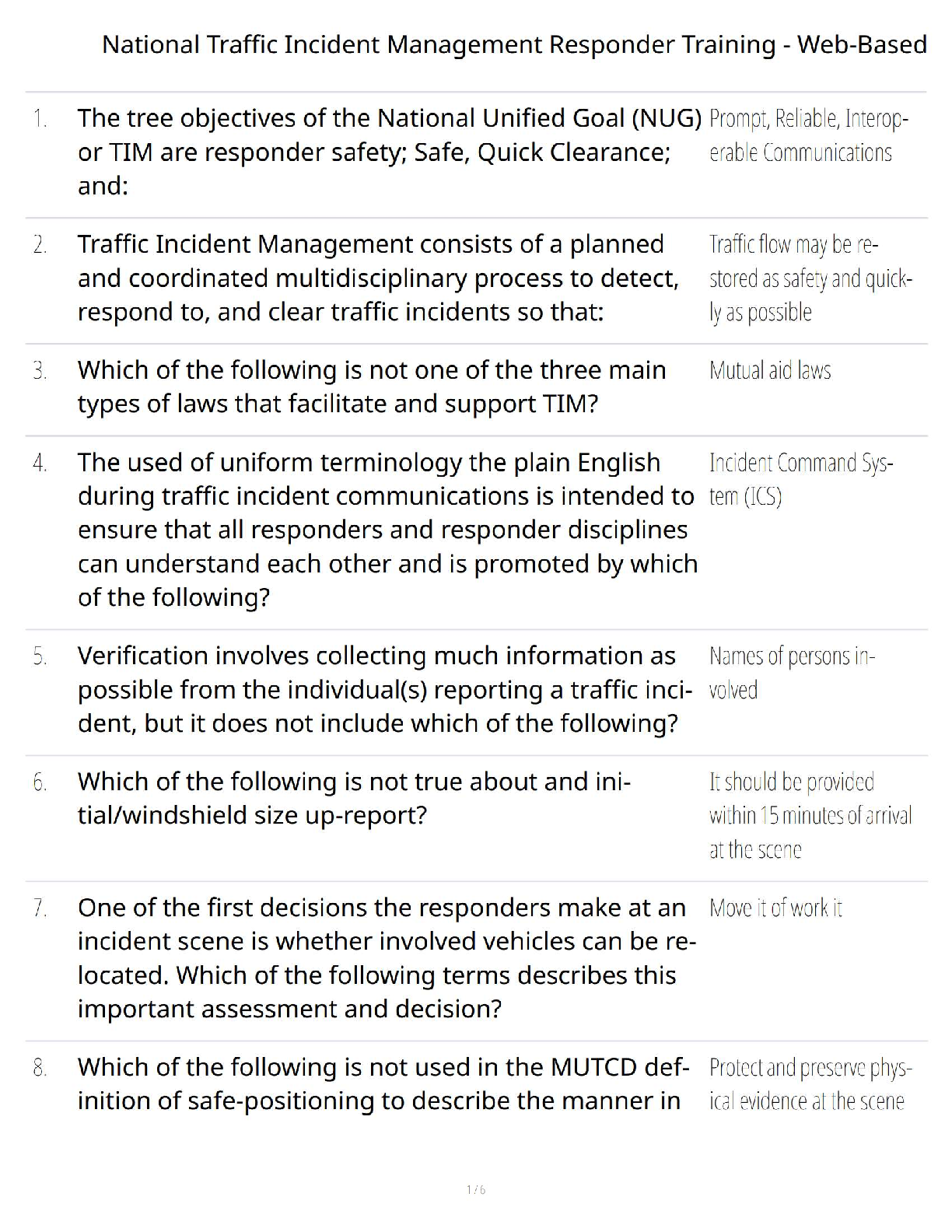
National Traffic Incident Management Responder Training - Web-Based
$ 12

Instructor Manual (Review Questions Answers) for Computerized Engine Controls 11th Editions By Steve Hatch, Tim Gilles, Cengage (All Chapters, 100% Original Verified, A+ Grade)
$ 20

PORTAGE LEARNING BIOD 151 ALL LAB EXAM QUESTIONS AND 100% CORRECT ANSWERS LATEST UPDATE 2024
$ 18

BIO -MARK SCHEME – GCSE COMBINED SCIENCE: TRILOGY – 8464/B/2H – JUNE 2020
$ 10

University of Maryland IFSM 201 Quiz 4
$ 10

Ivy Tech Community College of Indiana - NURSING Med Surg 2 SAUNDERS COMPREHENSIVE REVIEW FOR NCLEX ONE
$ 13.5

[eBook] [PDF] Contemporary Project Management Plan-Driven and Agile Approaches 5th Edition By Timothy Kloppenborg, Kathryn Wells, Vittal Anantatmula
$ 25
.png)
Tutorial Letter 104/3/2021 Database Practice ICT3722 Semesters 1&2 Computer Science
$ 15

AQA GCSE COMBINED SCIENCE: TRILOGY 8464/B/1H Biology Paper 1H Mark scheme June 2020 Version: 1.0 Final Mark Scheme
$ 10

Define chain of custody and explain the importance of maintaining proper chain of custody when
$ 7

Statistics Principles And Methods 8th Edition by Johnson, Bhattacharyya | INSTRUCTOR’S SOLUTIONS MANUAL
$ 19

Theories of Migration: Migration decision-making. Best Presentation
$ 5

Mobility Subjective Data. Medical Surgical Nursing: Promoting Wellness in the Older Adult (Los Angeles), NURS 201
$ 14
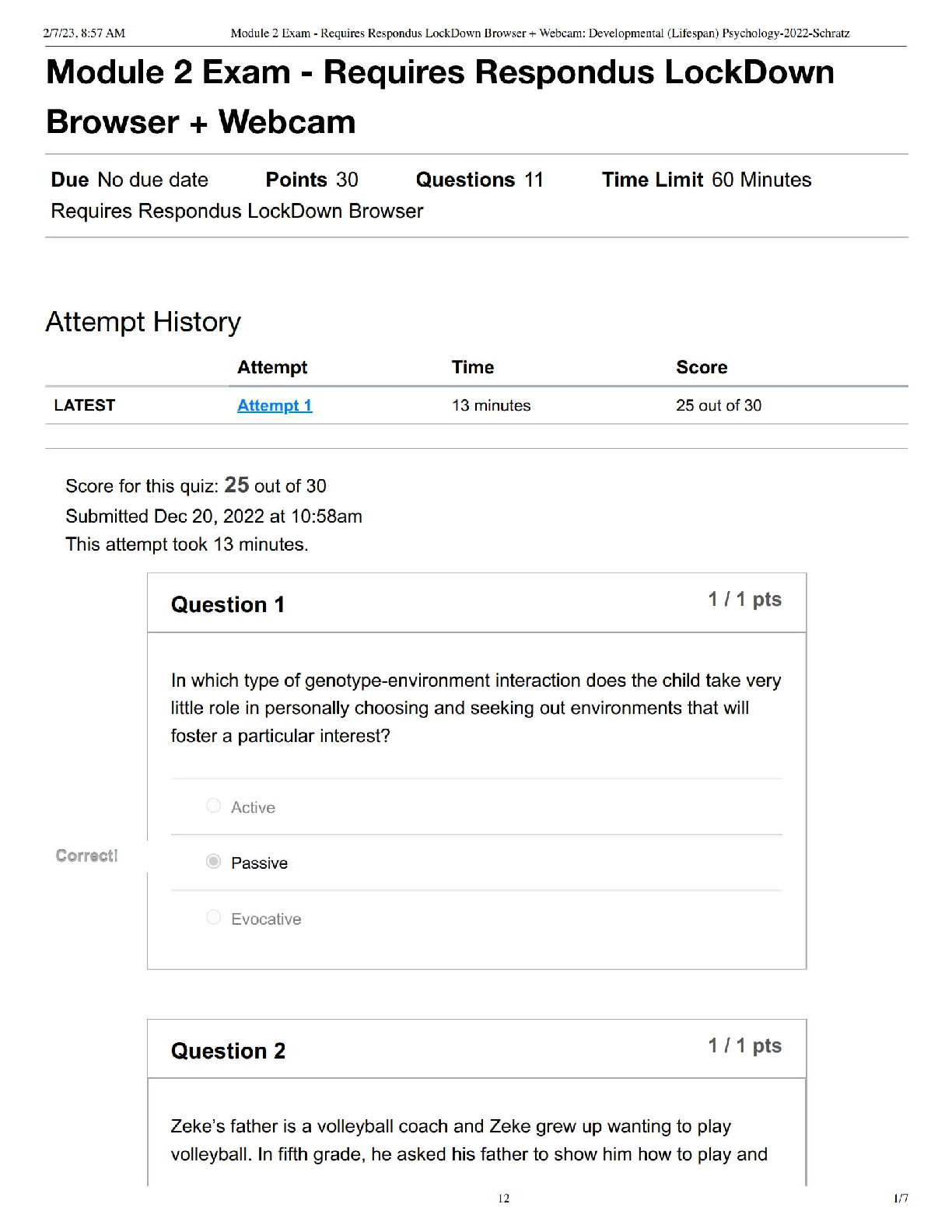
PSYC 140 (Developmental – Lifespan Psychology) Module 2 Exam | Requires Respondus LockDown Browser + Webcam – Verified Questions & Answers
$ 16.5

AP Psychology Unit 12 Outline: Abnormal Behavior MODULE 60: Introduction to Psychological Disorders
$ 3.5

2023 AANP Practice Questions (verified Q&A
$ 11
 Correct Study Guide, Download to Score A.png)
Introduction to Pharm, All Correct Latest Review, (Latest 2021) Correct Study Guide, Download to Score A
$ 24

Solutions Manual For A First Course In Machine Learning (2ND Edition) Exercise Solutions
$ 15.5

NSG 4028 : Concepts of learning week 1 quiz. South University
$ 8

Central Dogma and Genetic Medicine questions and answers grade A+
$ 12

VATI RN COMPREHENSIVE PREDICTOR REMEDIATION
$ 15

OCR GCE A LEVEL 2022 COMPUTER SCIENCE QUESTION PAPER H446-1 PAPER 1-H446/01 Computer Systems
$ 13

ANCIENT GREEK PHILOSOPHERS MILESTONE 1,100% CORRECT
$ 15

GIZMO: SCIENCE 1 Mouse Genetics (Two Traits).
$ 9
.png)
STR 581 Week 2 Capstone Final Examination Part 1
$ 8.5

Psychotherapy for the Advanced Practice Psychiatric Nurse, Second Edition: A How-To Guide for EvidenceBased Practice 2nd Edition Test Bank
$ 15.5

BIS 320 WEEK 2 IT PLANNING AND DATABASE PRESENTATION
$ 13

CWV 101 Topic 3 Quiz. Questions And Answers. Grand Canyon University.
$ 8
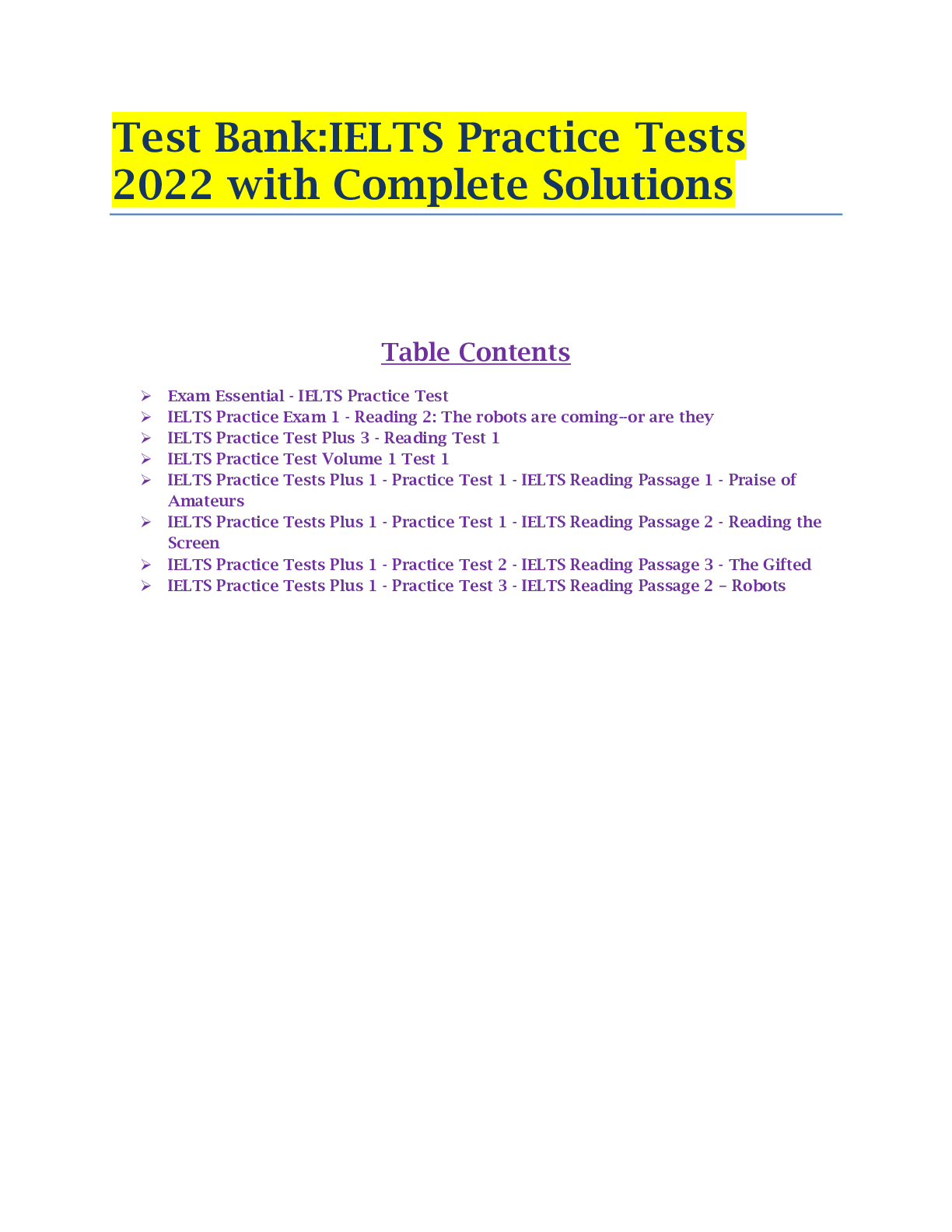
Test Bank:IELTS Practice Tests 2022 with Complete Solutions
$ 12

Comprehensive NCLEX NGN Practice Questions for FUNDAMENTALS OF NURSING
$ 31.5

CET 345 Materials Testing Laboratory: Verification of Testing Machine, full lab report
$ 10

Forensic Sculpting / Techniques, Certification & History / Exam Prep 2025 / Score 100% Study Guide & Test Bank
$ 24

Instructor’s Manual: Exercise Solutions for Artificial Intelligence A Modern Approach Fourth Edition
$ 15

Test Bank For Fluid Mechanics Fundamentals and Applications 4th Edition By Yunus Cengel, John Cimbala | All Chapters, 100% Original Verified
$ 23

NR 327 OB exam 1 review Best Study Notes
$ 10
.png)
HEALTH PROMOTION
$ 11.5

NR508 Midterm Exam Study Guide |With Absolute Complete And Latest Solutions
$ 15

MSN 570 FINAL EXAM ADVANCED PATHOPHYSIOLOGY 2023
$ 24

PRINCIPLES OF SURGERY EXAM ANSWER KEY 2024-COMPLETE SOLUTION(102 Questions)
$ 14.5

LAW604 Midterm 1 Review
$ 6

CWV 101 Final Exam. Questions And Answers 2022.
$ 12.5

HESI FUNDAMENTALS 2024/2025 EXAM STUDY QUESTIONS WITH VERIFIED ANSWERS GUARANTEED PASS | RATED A+
$ 15

SAUNDERS COMPREHENSIVE REVIEW FOR NCLEX | 1140 Practice Test Best Solution to Ace Your Exam
$ 14

PHIL 347 Week 5 - Course Project; Annotated Bibliography and Source Evaluation - Immigration
$ 6

GCE Computer Science H446/01: Computer systems A Level Mark Scheme for June
$ 10.5

Solutions Manual For Anatomy & Physiology An Integrative Approach 1st Edition By Michael McKinley Valerie O'Loughlin Theresa Bidle (All Chapters, 100% Original Verified, A+ Grade)
$ 18

relab Exercises Question 1 When discharging, what percentage of the starting voltage will the capacitor reach after 5RC? Answer 1 Question 2 How many time constants would you have to wait before the voltage across the capacitor is within 99% of its final voltage? Answer 2 Question 3 When the lightbulbs were used as resistors, you observed only a flash of light, as opposed to a continuous glow. Explain why that behavior is expected. After all, the light bulb is directly connected to the power supply. Answer 3 Question 4 Which combination will cause the fastest charging rate? Answer 4 Question 5 Which combination will cause the slowest charging rate?
$ 5
.png)
LAW ON OBLIGATIONS (Latest 2021) 100% Correct Study Guide, Download to Score A
$ 15

MORALS STATUS
$ 10

Ohio MPJE | 470 Questions with 100% Correct Answers
$ 10
.png)
Florida Atlantic University - BCH 3033Biochem Final Exam StudyGuide. This document contains over 200Q&A, With Correct answers Indicated in bold.
$ 10

C785 Biochemistry Module 2 Quiz 2020 | C 785 Biochemistry Topic 2 Quiz 2020– Western Governors University
$ 13.5

AQA GCSE COMBINED SCIENCE: TRILOGY 8464/B/2H Biology Paper 2H Mark scheme June 2020 Version: 1.0 Final
$ 8

LEHNES PHARMACOTHERAPEUTICS FOR ADVANCED PRACTICE NURSES AND PHYSICIAN ASSISTANTS 2ND EDITION ROSEN
$ 22

Frank Wood’s Business Accounting 1 & 2 ELEVENTH EDITION: THE SOLUTION MANUAL
$ 11

C785 Biochemistry Module 1 Quiz 2020 | C 785 Biochemistry Topic1 Quiz 2020 – Western Governors University ( Download to Score A)
$ 14

NURSING 301ati remediation community.rtf
$ 7.5

Pearson Edexcel_Religious Studies_9RS0/4D Mark Scheme_2021 | In Religious Studies - Paper 4D: Islam
$ 6.5

NUR1211 - Keiser University - UWorld Review: Must-Have Study Notes for the NCLEX
$ 15
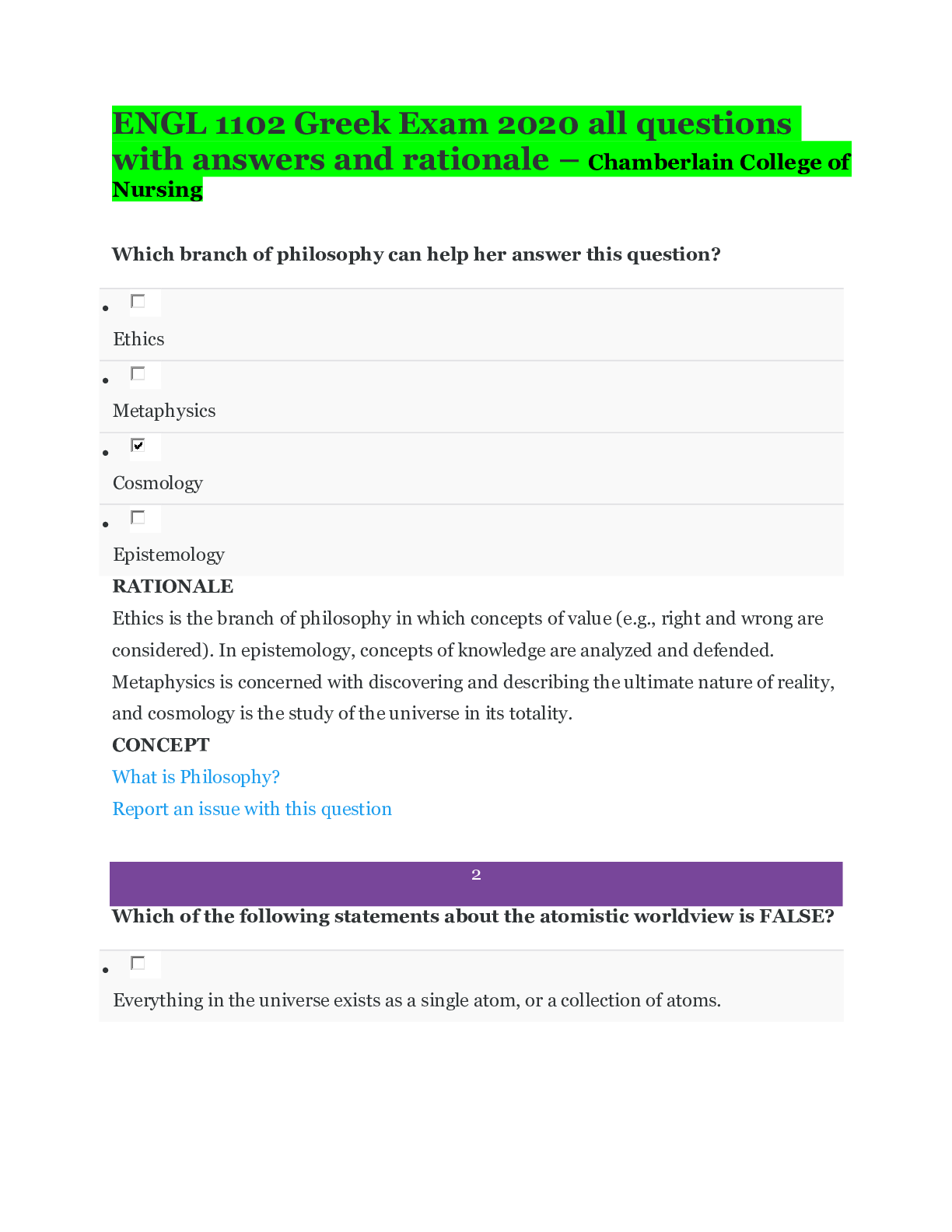
ENGL 1102 Greek Exam 2020 all questions with answers and rationale – Chamberlain College of Nursing
$ 12.5

solutions manual for engineering hydrology 3rd edition by dr.k. subramanya
$ 9

ATI MANAGEMENT ONLINE PRACTICE 2020 B REVISED.
$ 15

NCLEX 3500 / Score 100% / 2025 Update / Nursing Exam Test Bank & Study Guide
$ 14
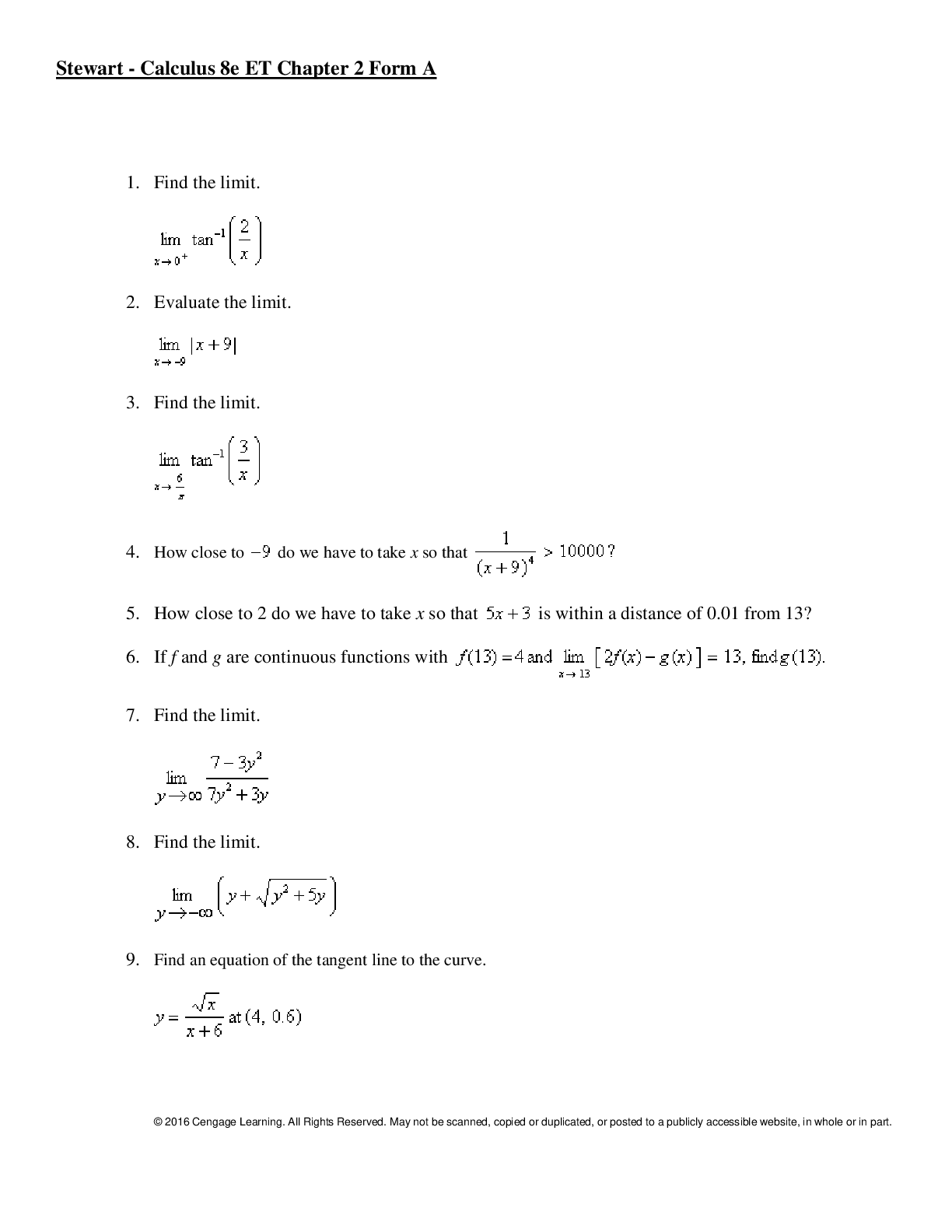
Stewart - Calculus 8e ET Chapter 2. All Answers
$ 8

NSG 6420 - Final Review.
$ 10

CHANGE THEORY PROJECT UNIVERSITY OF TEXAS- ARLINGTON RN-BSN NURSING MANAGEMENT-INFECTION CONTROL
$ 13

Developing Leaders - LeadingMarines TEST 100%CORRECT SOLUTIONS2023-2024
$ 10
Pearson Edexce Time 55 minutes History PAPER 2: Period Study Paper reference 1 HIA/P5 Option P5: Conflict in the Middle East, 1945-95
$ 7

UNIV_1001_Final_Exam. LATEST 2021/2022
$ 14

ATI ELSEVIER HESI MEDICAL SURGICAL EXAM STUDY GUIDE FOR - DISEASE MANAGEMENT I - SECONDARY PREVENTION -ACUTE CARE 2023
$ 14.5

C H A P T E R 1 7 Theories of Growth and Development: From Saunders Comprehensive Review for the NCLEX-RN Examination 8th Edition. (Available: https://bit.ly/2HeJuMt ). Contains Practice questions and Answers with the Rationale, Test-Taking Strategy, Level of Cognitive Ability, Client Needs, Integrated Process, Content Area, Health Problem, Priority Concepts and References
$ 3

(Capella) NURS6105 Teaching & Active Learning Strategies Comprehensive Exam Guide Q & A 2024
$ 12

(Answered) Week 5 Introduction to Visual Literacy Quiz Retake, all corrected
$ 16

NSG 5003 PATHO TEST ONE with all the correct answers,latest update 2020
$ 5

WGU C955 - Module 4: Descriptive Statistics for a Single Variable(Answered)2022/2023
$ 10.5

RN Adult Medical Surgical Online Practice 2019 A | Adult Medical Surgical Online
$ 14.5

[eTextBook] [PDF] The Analysis and Design of Linear Circuits 10th Edition By ROLAND THOMAS, ALBERT ROSA, GREGORY TOUSSAINT
$ 29

NURS 6551 Midterm Exam | How can liver and renal diseases result in abnormal uterine bleeding (AUB)?