Biology > QUESTIONS & ANSWERS > AQA A Level Biology Unit 1 Questions and Answers Rated A+ (All)
AQA A Level Biology Unit 1 Questions and Answers Rated A+
Document Content and Description Below
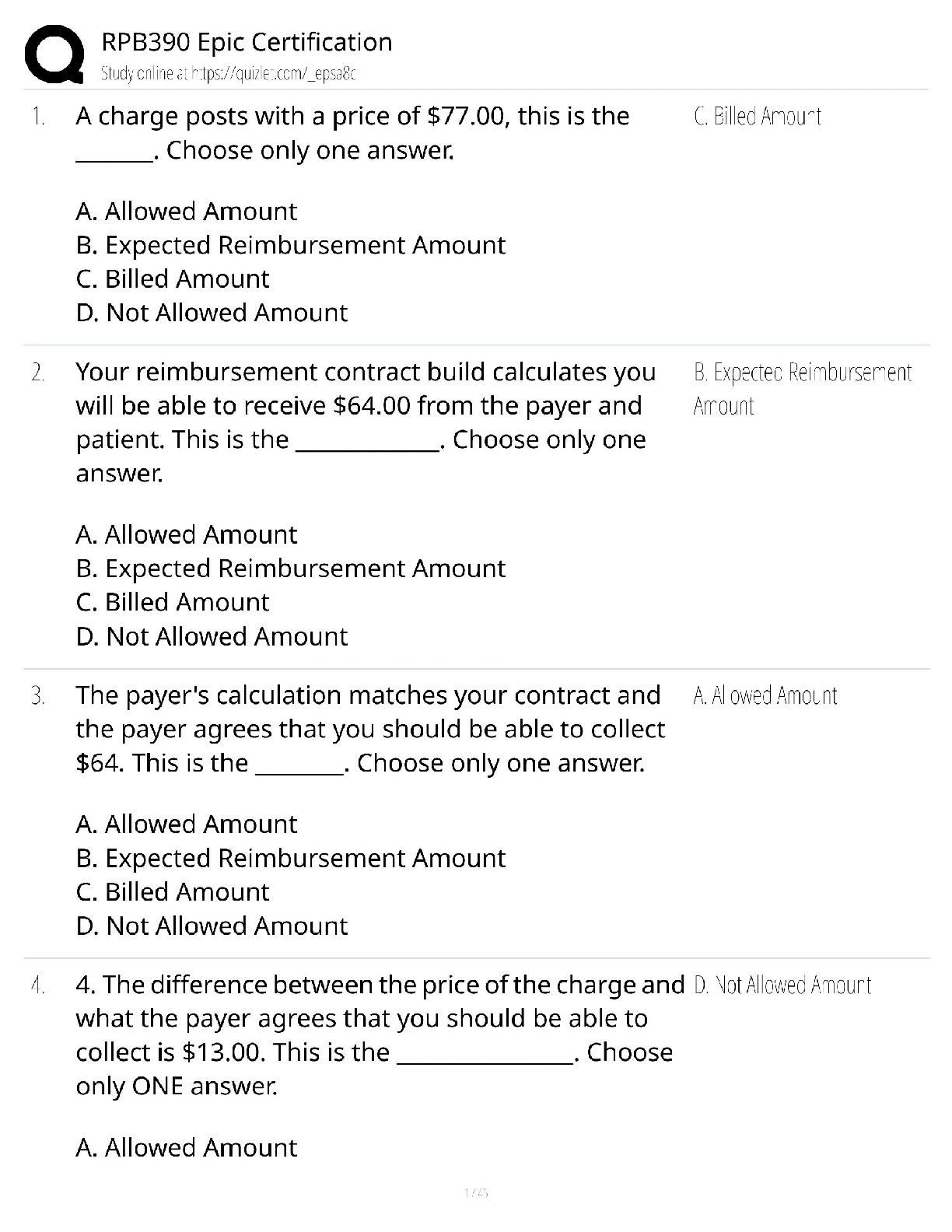
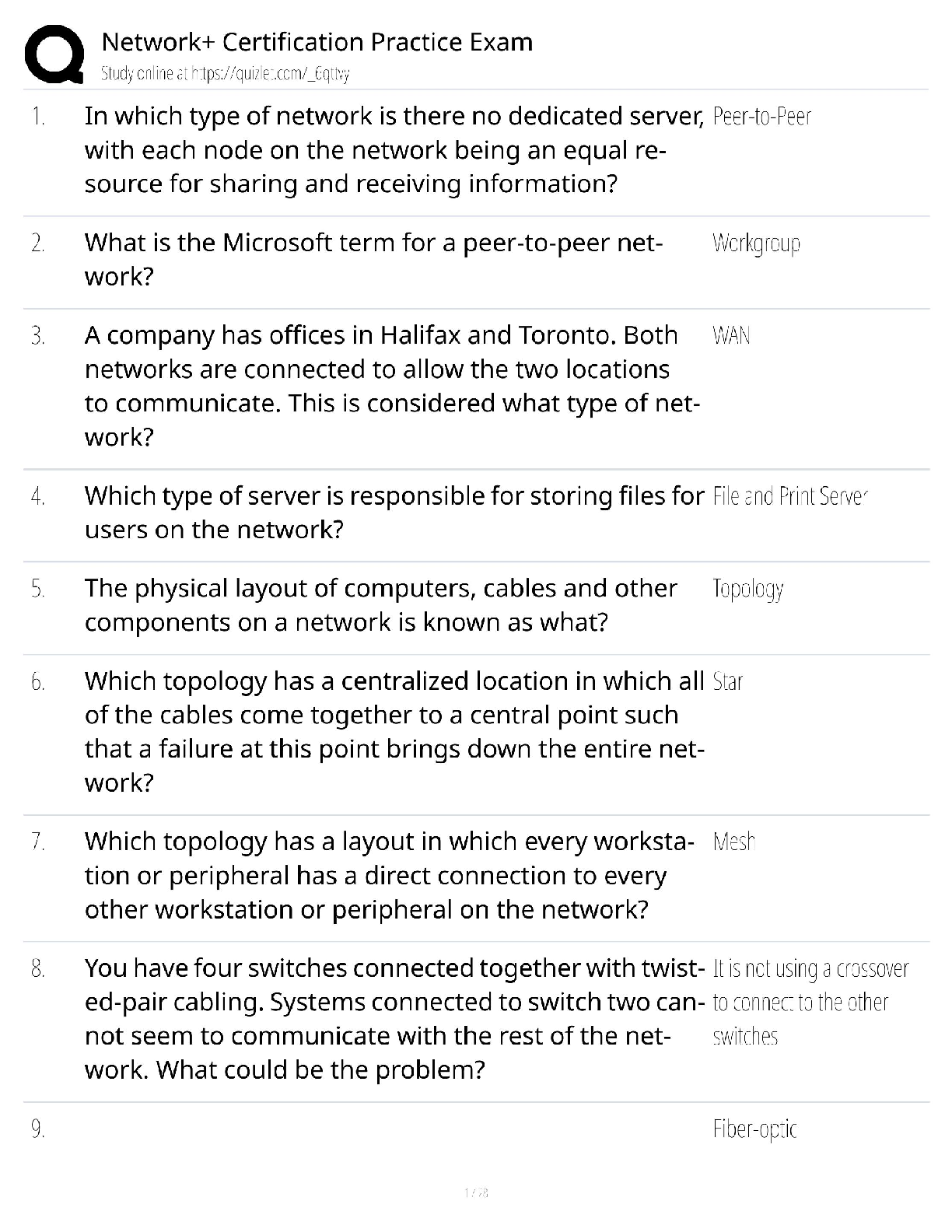
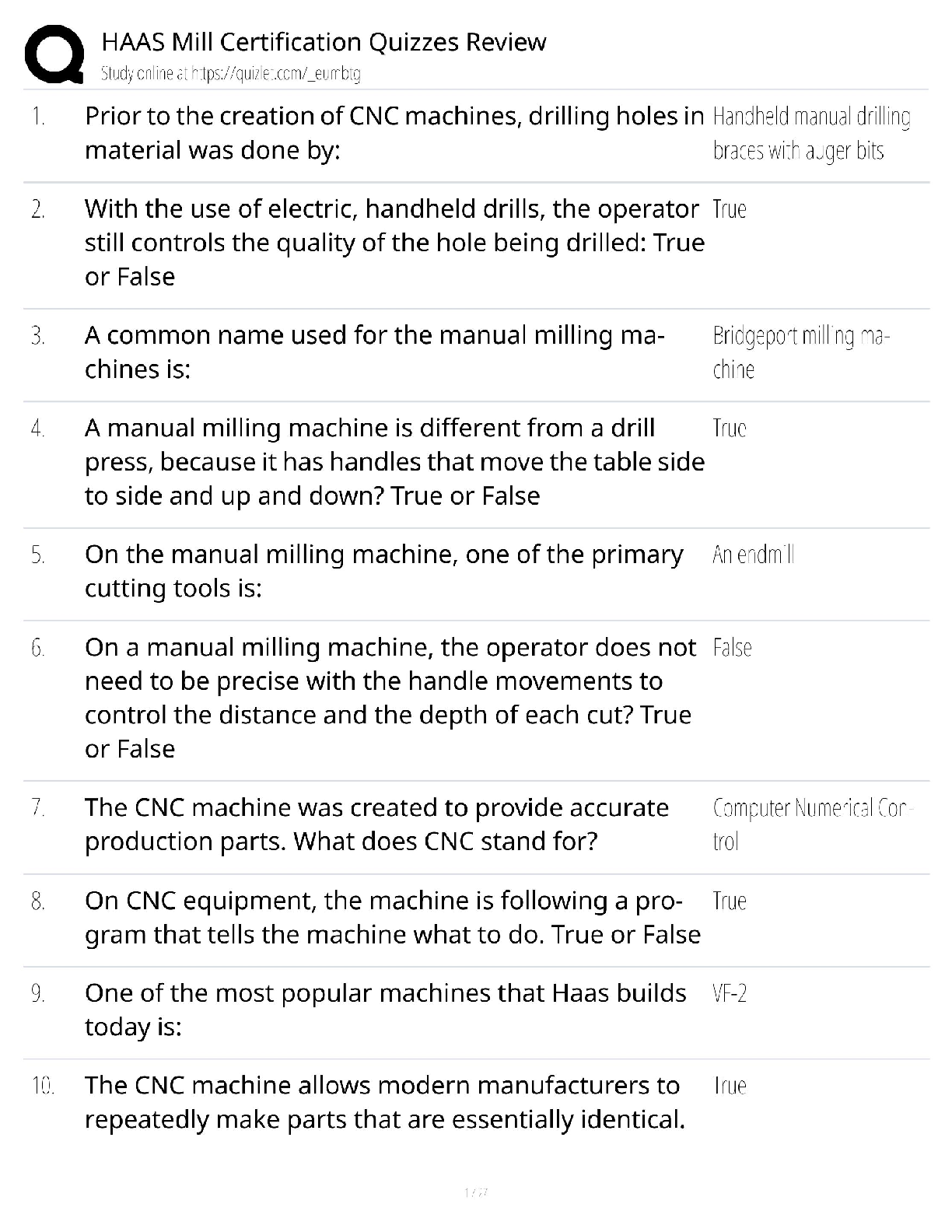
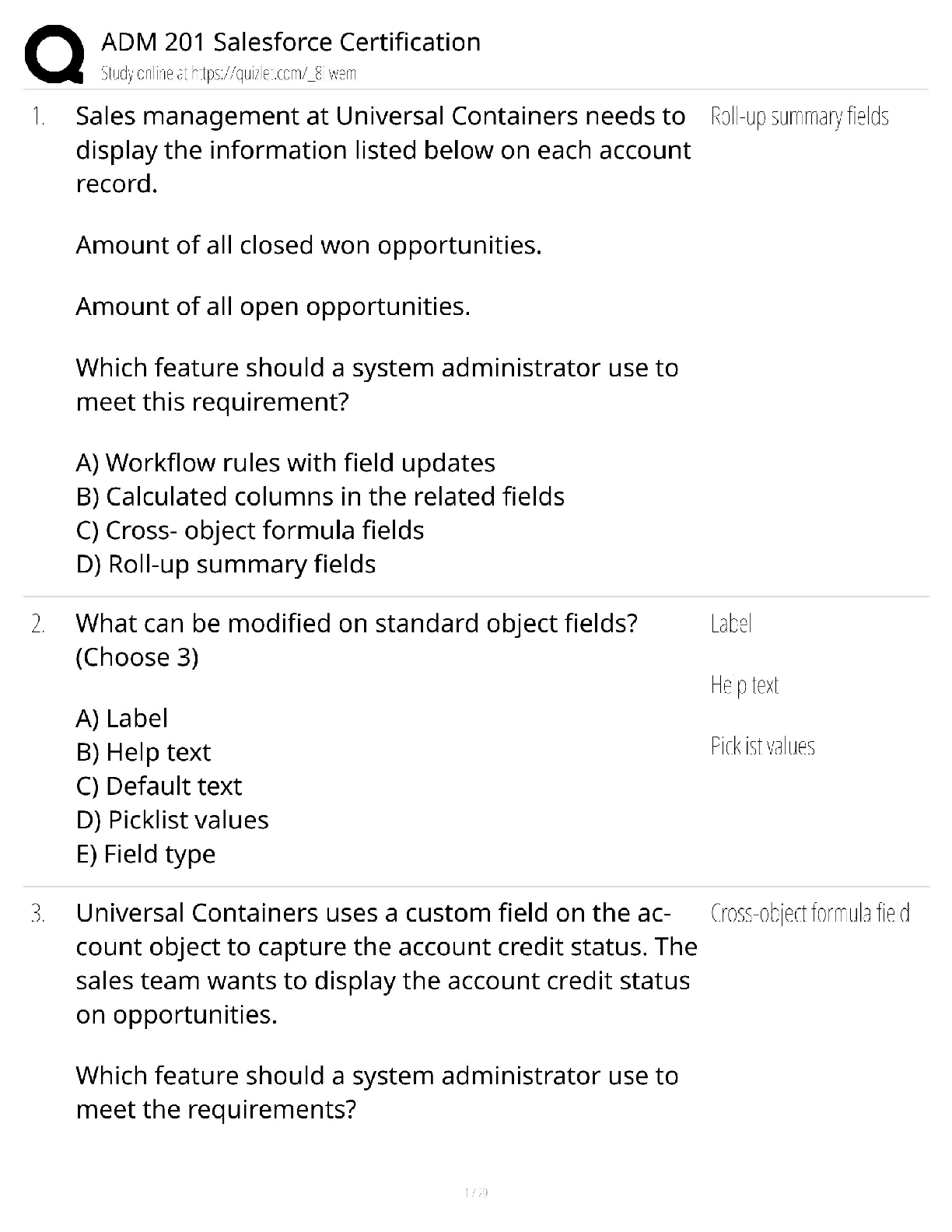
AQA A Level Biology Unit 1 Questions and Answers Rated A+ How do biological molecules provide evidence for evolution? They all have similar molecules and use the same amino acids and DNA. What are ... monomers of carbohydrates called? Monosaccharides. Beta Glucose Alpha glucose Glucose formula C₆H₁₂O₆ Name of the bond between monosaccharides? Glycosidic bond. What is sucrose made from? Glucose and fructose. What is lactose made from? Glucose and galactose. What is maltose made from? Two alpha glucose. Testing for reducing sugars? Add Benedict's reagent (blue) and heat in a water bath at a boil. If present: Green → yellow → orange → brick red Test for non-reducing sugars? Add dilute hydrochloric acid and heat, neutralise with Sodium Hydrogencarbonate and add Benedicts reagent. What is the scientific name for carbohydrates? Polysaccharides. What is starch made of? Starch is a mixture of two polysaccharides of alpha glucose: Amylose and amylopectin. What is amylose? A long, unbranched alpha glucose chain. The angles in it make it have a coiled shape which makes it compact and effective for storage. What is amylopectin? A long, braqnched alpha glucose chain. The side branches mean that enzymes can get to and hydrolise glycosidic bonds faster so glucose can be released quickly. What is glycogen? A polysaccharide of alpha glucose with a similar structure to starch except it is more branched, allowing even faster energy release. It is also very compact. What is celluose? it is made of long. straight chains of beta glucose. The chains are linked my hydrogen bonds to form microfibrils, a strong fibre. The strong fibres allow it to provide structural support in cells as cell walls. How to test for starch. Add iodine which is dissolved in potassium iodie ti the sample. Positive result: Turns blue-black. Negative result: stays browny-orange. Which of proteins, polysaccharides and lipids are not a polymer. Lipids. What are triglycerides made of? Glycerol and fatty acids. What causes lipids to be insoluble in water? The hydrophobic fatty acid tails. What is the structure of a fatty acid? What do double bonds cause in fatty acids? A kink in the chain. Triglyceride formation? What are phospholipids? Similar to triglycerides, but one of the fatty acid tails is replaced by a phosphate group head. Which part of a phospholipid is hydrophillic and which part is hydrophobic? The phosphate group is hydrophillic and the fatty acid tail is hydrophobic. What are triglycerides mainly used for? To store energy, they are effective at this as the hydrocarbon tails contain a lot of energy. They are also insoluble so do not affect the water potential of cells, preventing swelling. How much energy do lipids contain compared to carbohydrates? Twice as much energy per gram. Why do triglycerides bundle together in water? The fatty acid tails are hydrophobic so face inwards, so are shielded by the glycerol heads. What role do phospholipids perform? They make up the bilayer in cell membranes. Test for lipids? The emulsion test: Shake substance with ethanol and pour into water. A white emulsion forms if it is present. What are proteins made of? Amino acids. What is the bond between amino acids called? Peptide bond. Amino acid structure. Amino group, variable group, carboxyl group. What is a primary structure? Polypeptide chain. What is a secondary structure? Chain twists into alpha helix or folds in beta pleated sheet. What is a tertiary structure? Chain coils further, forming hydrogen bonds, ionic bonds and disulphide bridges. When do disulphide bridges form? When the sulphur atoms on the amino acid cysteine form sulphur bonds. What is a Quaternary structure> Like tertiary, but made of several chains. Test for protein? Biuret test. Add a few drops of Sodium Hydroxide, then add copper sulphate. Purple positive. Blue negative. What is formed when catalysts join to substrates? Enzyme-substrate complex. What does DNA stand for? Deoxyribonucleic acid. What does RNA stand for? Ribonucleic acid. Nucleotide structure. What is the monomer of DNA and RNA? Nucleotides. The polymer is called Polynucleotide strands. What are the sides of DNA and RNA called? Sugar-phosphate backbone. What is the name for the bond between the pentose sugar and phosphate group of nucleotides? Phosphodiester bond. Why can a lot of genetic information fit in the nucleus? DNA molecules are long and coiled tightly. What are the base pairings? Adenine - thymine (two bonds) Cytosine- Guanine (three bonds) How are bases in DNA joined? Hydrogen bonds. How are the polynucleotide strands in DNA arranged? They are antiparallel. One runs from 3' to 5' and the other 5' to 3'. What did scientists originally think carried genetic information? Proteins. They thought DNA was too simple. How does DNA replicate? Semi-conservatively. One original strand goes to each new cell. What does semi-conservative replication result in? Genetic continuity between generations of cells. The process of DNA replication. 1) DNA helicase breaks hydrogen bonds, unravelling DNA. 2) Each strand acts as a template, complementary base pairing means nucleotides are attracted. 3) Condensation reactions join nucleotides of new strands. together, caused by DNA polymerase 4) Hydrogen bonds form between bases. What is the difference between the 3' and 5' end of DNA? 3' has a hydroxyl (OH) group on the pentose sugar and 5' end is a phosphate group. How does DNA polymerase move and add nucleotides? It moves from a 3' to a 5' end as the active site is only complementary to the 3' end of the DNA strand. This means nucleotides are added in a 5' to 3' direction. Details of the Meselson and Stahl experiment. 1) Two samples of bacteria grown in ¹⁵N and ¹⁴N nutrient broth. Bacteria took up N in replication. 2) DNA samples taken, spun in centrifuge. Heavy N settled further down. 3) DNA from ¹⁵N placed n ¹⁴N for one replication. and spun in centrifuge. 4) Only one line formed in middle so semi- conservative. What does ATP stand for? Adenosine triphosphate. In what process is ATP produced? Respiration, made from glucose. Structure of ATP. How is ATP broken up? Broken by hydrolysis, a phosphate bond (the end which has the most) is broken which releases energy. Produces ADP and an inorganic phosphate group. Catalysed by ATP hydrolase. How can ATP hydrolysis be used? Coupled to energy requiring reactions, meaning energy can be used to make reaction occur, rather than wasted as heat. How can the inorganic phosphate produced in ATP hydrolysis be used? Phosphorylation. This is adding it to another compound, making it more reactive. How can the ADP produced in ATP hydrolysis be used? Can be recycled by joining with inorganic phosphate in a condensation reaction to form ATP. Occurs in respiration and photosynthesis. Catalysed by ATP synthase. What are important functions of H2O? 1) Important metabolite for condensation and hydrolysis. 2) Solvent, substances can dissolve, most reactions are in solution. 3) High specific heat capacity and latent heat of vaporisation, allows for temperature control. 4) Cohesive so stick together- useful for xylem. What is an inorganic ion? An ion with no carbon. [Show More]
Last updated: 2 years ago
Preview 1 out of 10 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

AQA A-Level Biology Bundled Exams Questions and Answers with Verified Solutions
AQA A-Level Biology Bundled Exams Questions and Answers with Verified Solutions
By Nutmegs 2 years ago
$23
21
Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 29, 2023
Number of pages
10
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 29, 2023
Downloads
0
Views
335








.png)