CHEMISTRY 111Lab 9.4.4
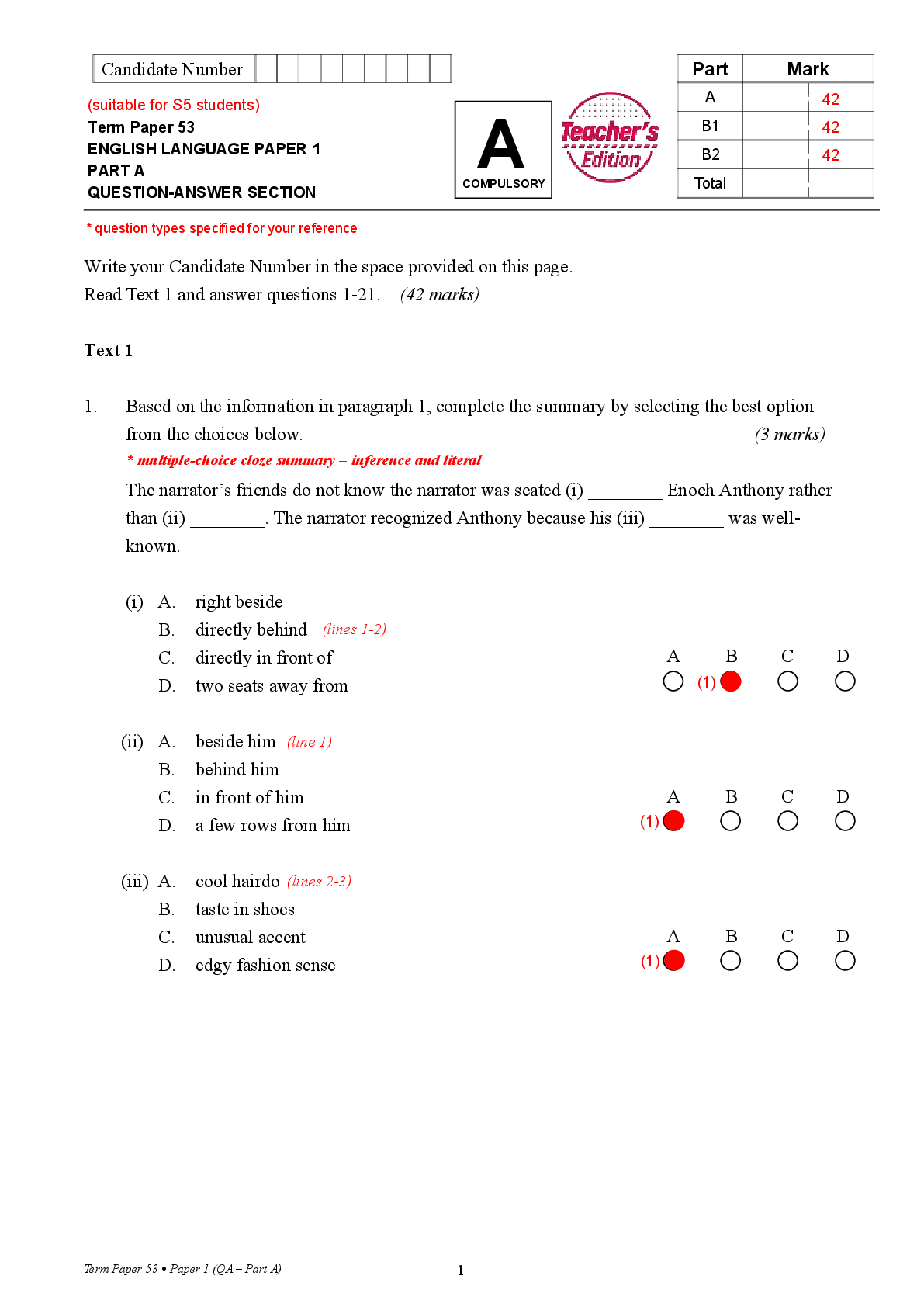
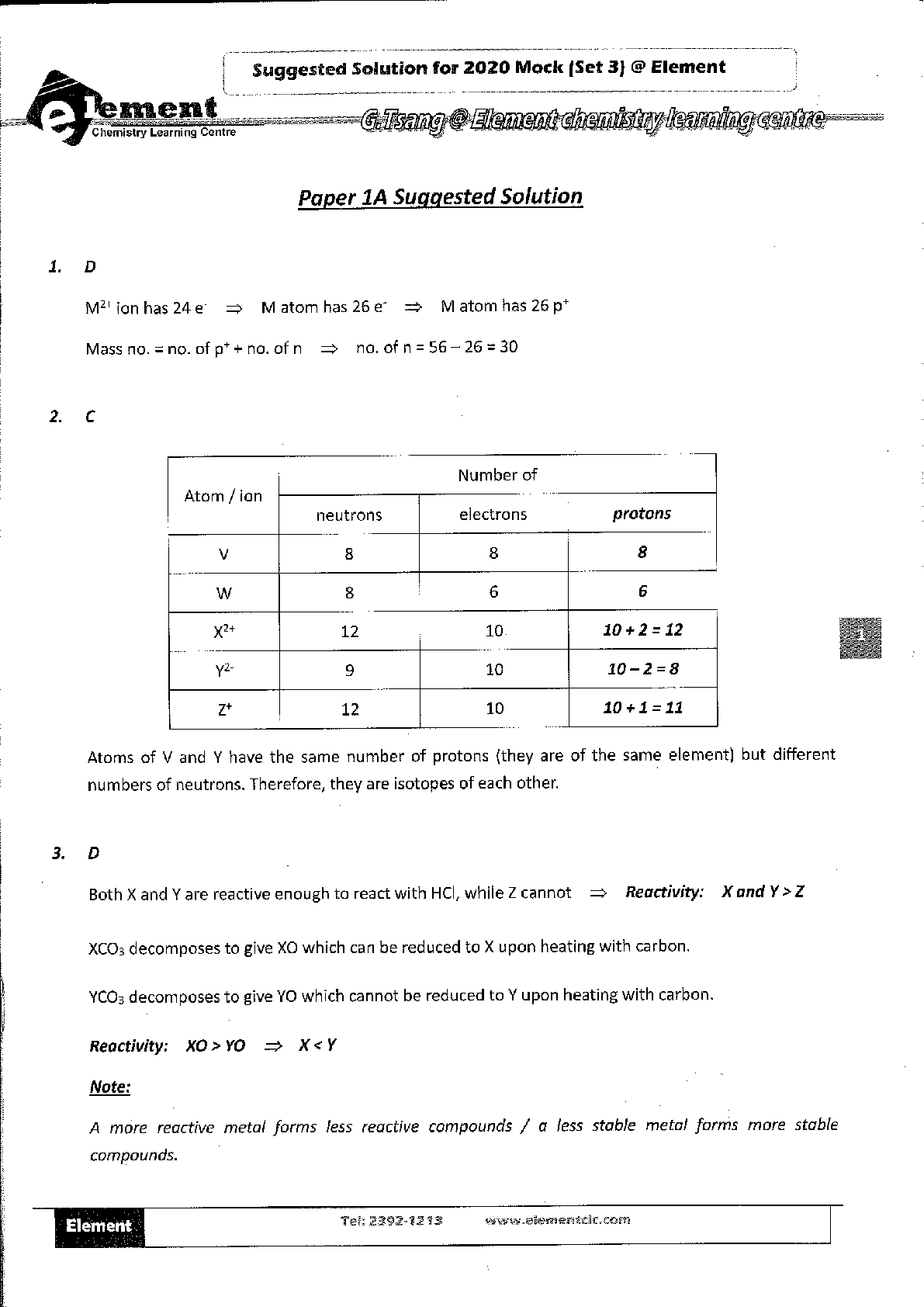
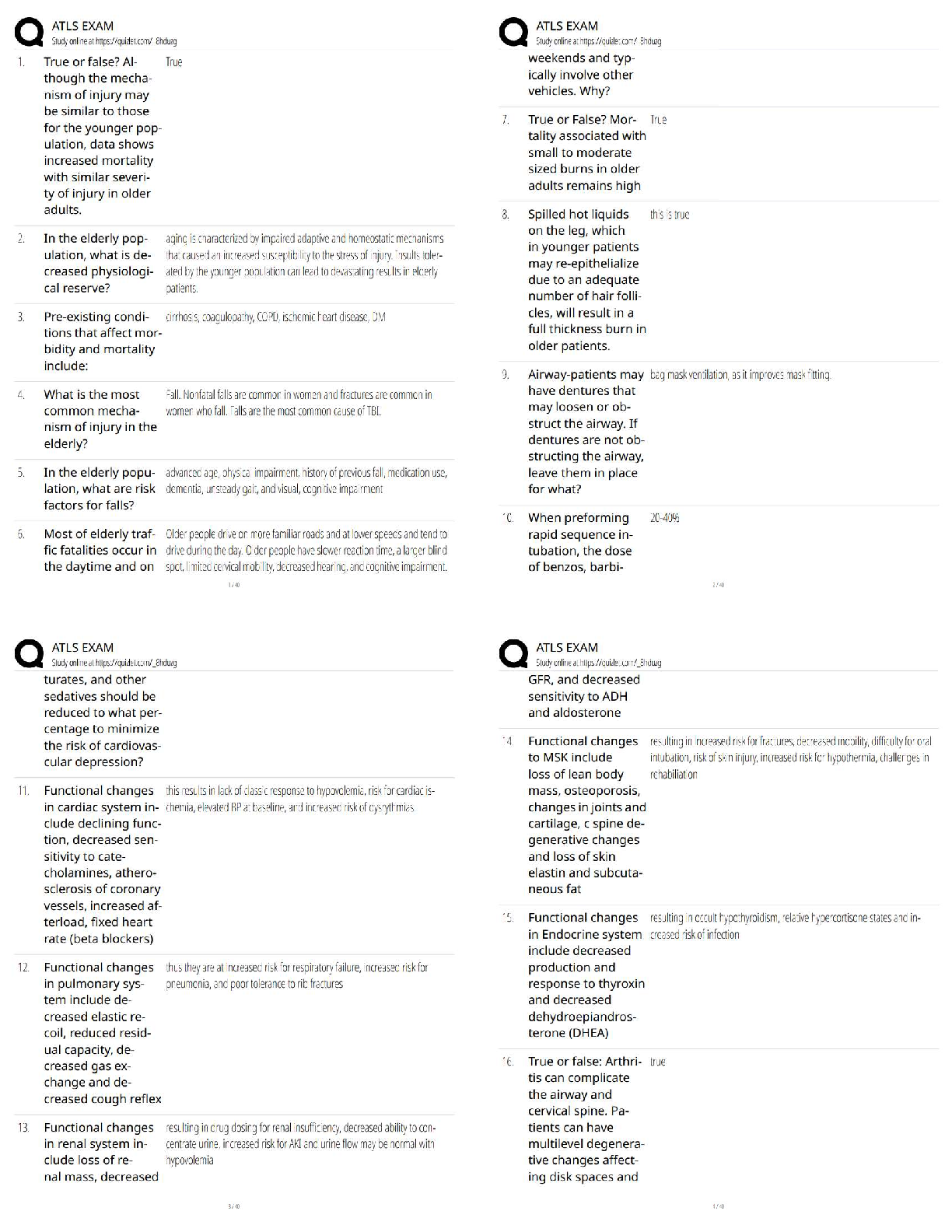
1. Suppose you wanted to calculate the heat of reaction for the formation of ammonia gas and gaseous hydrochloric acid (both of which are potentially dangerous in gaseous form) from solid ammoni
...
CHEMISTRY 111Lab 9.4.4
1. Suppose you wanted to calculate the heat of reaction for the formation of ammonia gas and gaseous hydrochloric acid (both of which are potentially dangerous in gaseous form) from solid ammonium chloride.
a. Write a balanced equation for this reaction.
b. The heat of reaction for the process described in (a) can be determined by applying Hess's law. The heats of reaction shown in the table below can be obtained experimentally or looked up in tables of enthalpy data. Which two of these heats of reaction would be the easiest and safest to measure in the laboratory, and which two are better obtained through reference sources? Why? Hint: Consider whether a reaction takes place in aqueous solution or instead involves noxious
c) What is the heat of reaction for the process described in (a)? Hint: Apply Hess's law using the data in (b).
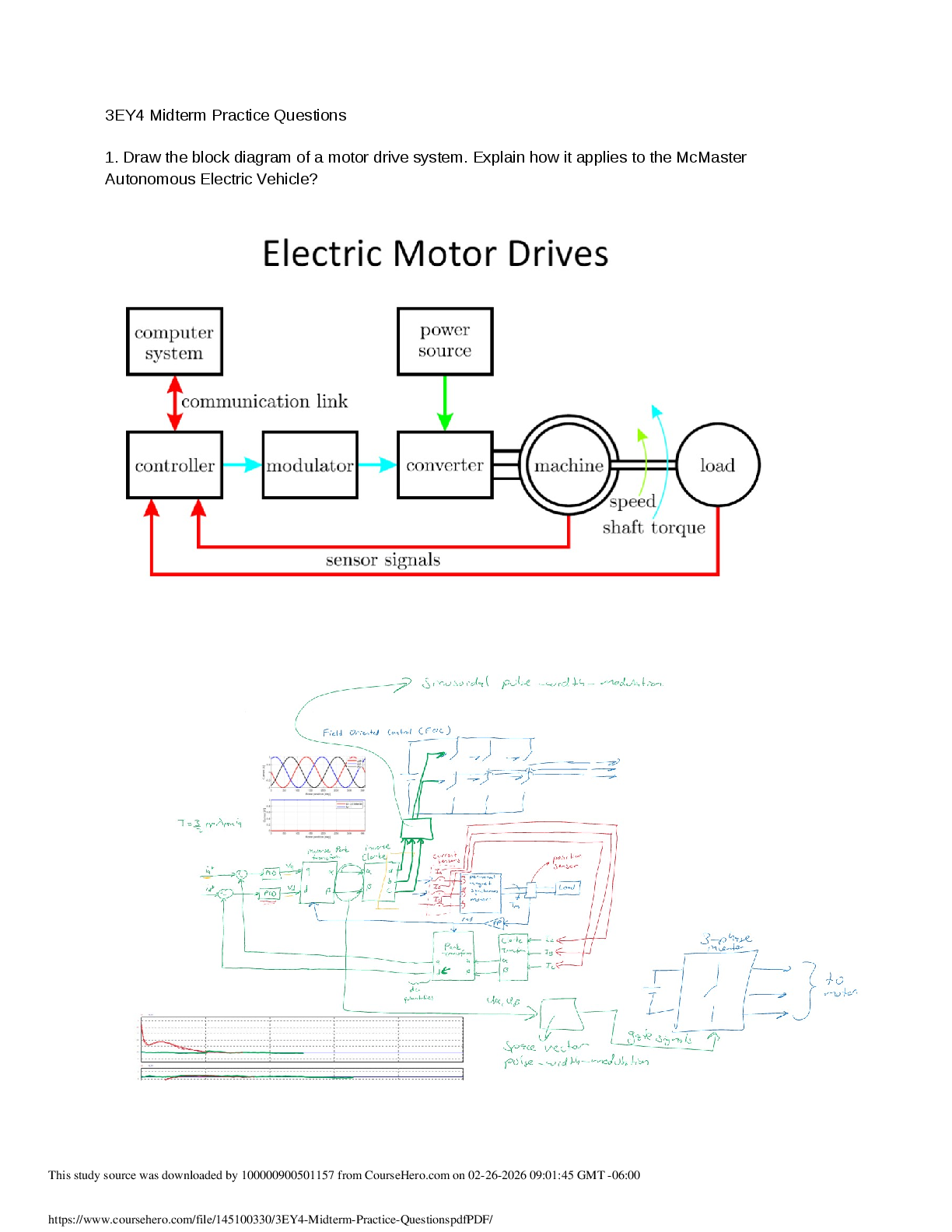
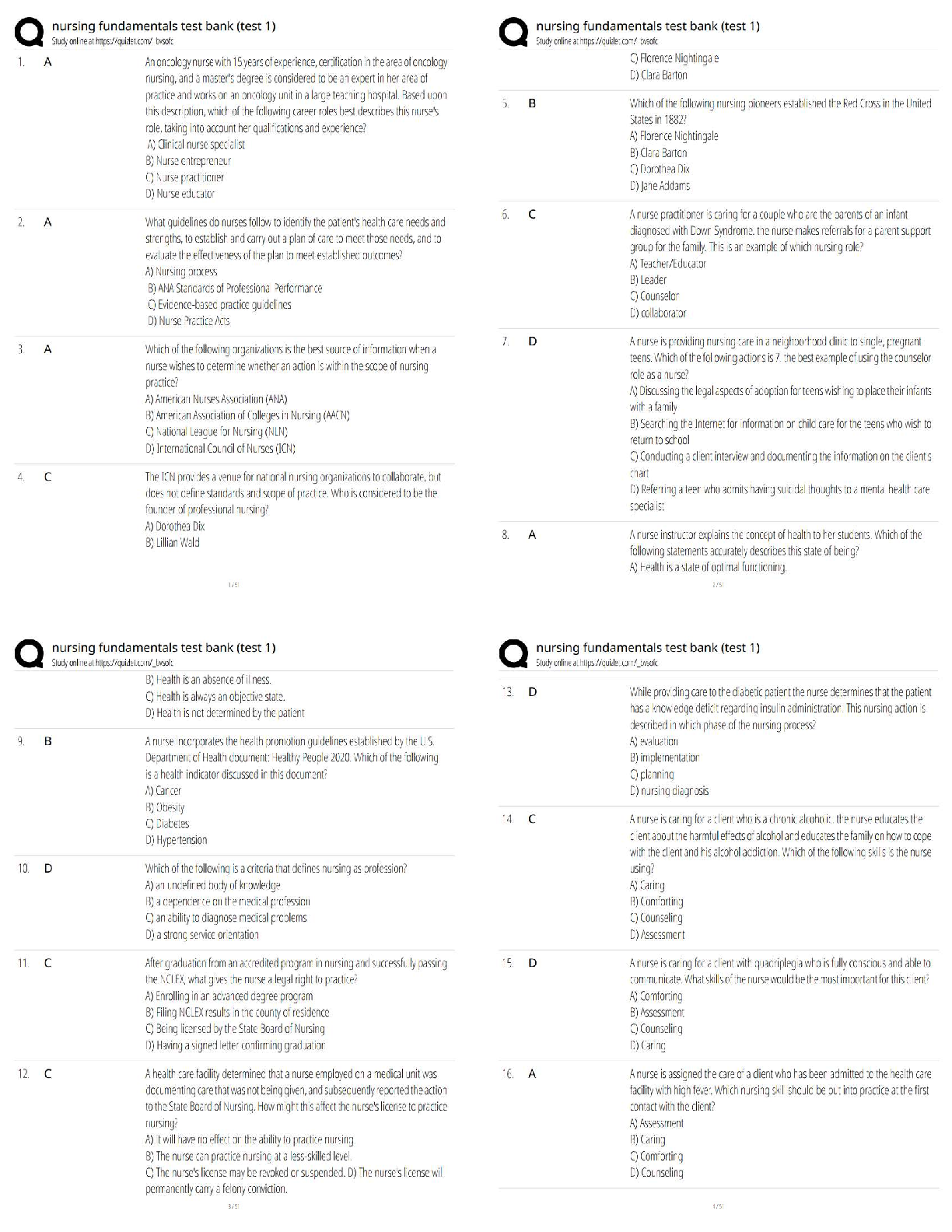
1. Calculate the approximate enthalpy of the reaction in joules. Estimate that 1.0 mL of vinegar has the same thermal mass as 1.0 mL of water. Ignore the thermal mass of the sodium bicarbonate. Note: It takes about 4.2 joules (J) to change 1.0 gram (1.0 mL) of water 1.0 °C.
2. Is the reaction endothermic or exothermic?
3. What steps could be taken to improve the accuracy of your results? The accuracy could be improved with a better calorimeter, better thermometer, and a larger sample.
4. Did the temperature go up or down? How much?
5. Is this an exothermic or endothermic reaction?
6. Think of some other chemical reactions. List them and the type of reaction: exothermic or endothermic.
Example: Burning coal – exothermic
[Show More]