Geology > QUESTIONS & ANSWERS > Questions and Answers > GEOL 3041/ GEOL 3041 Igneous & Metamorphic Petrology/Answers for Chapter 6: (All)
Questions and Answers > GEOL 3041/ GEOL 3041 Igneous & Metamorphic Petrology/Answers for Chapter 6: The Phase Rule and One-Component Systems.
Document Content and Description Below
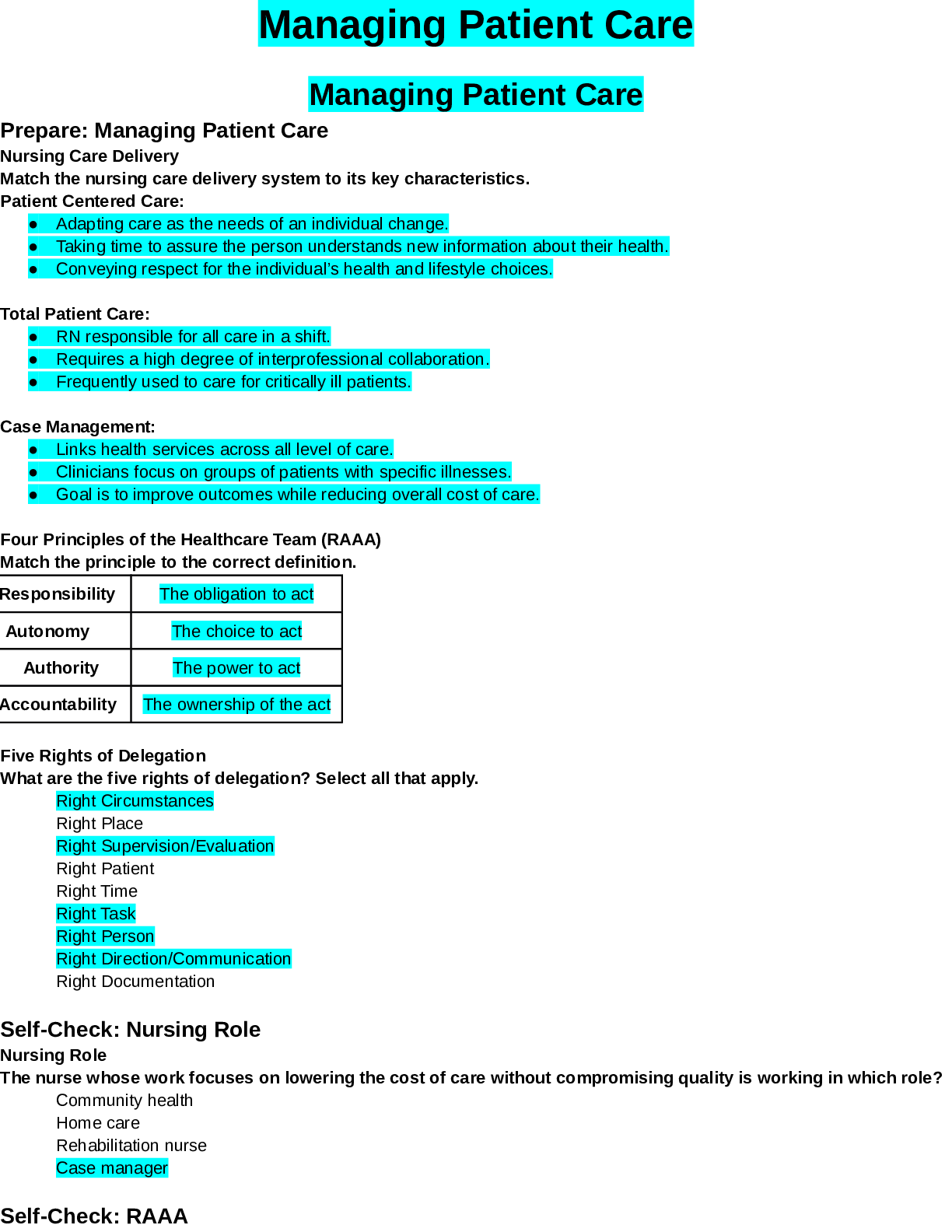
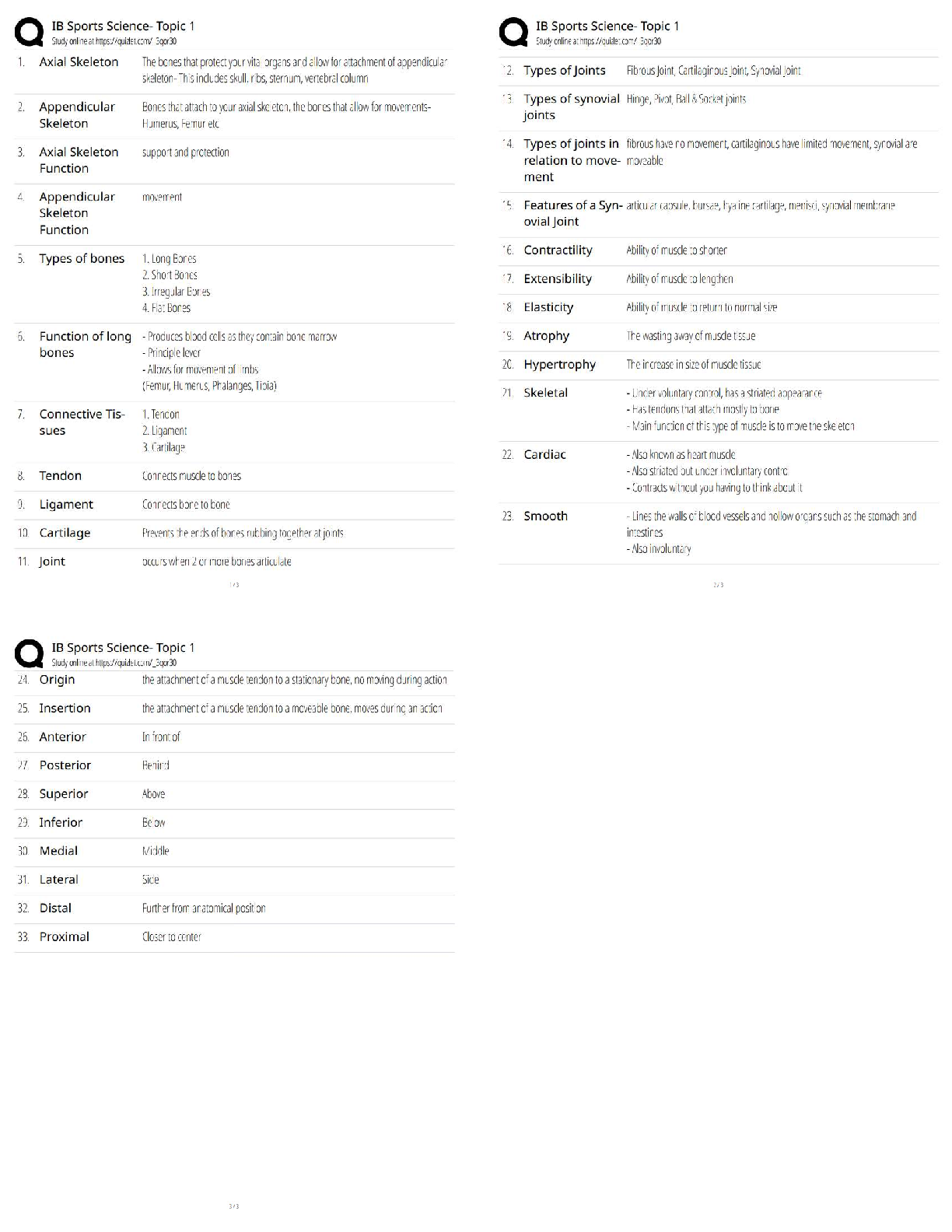
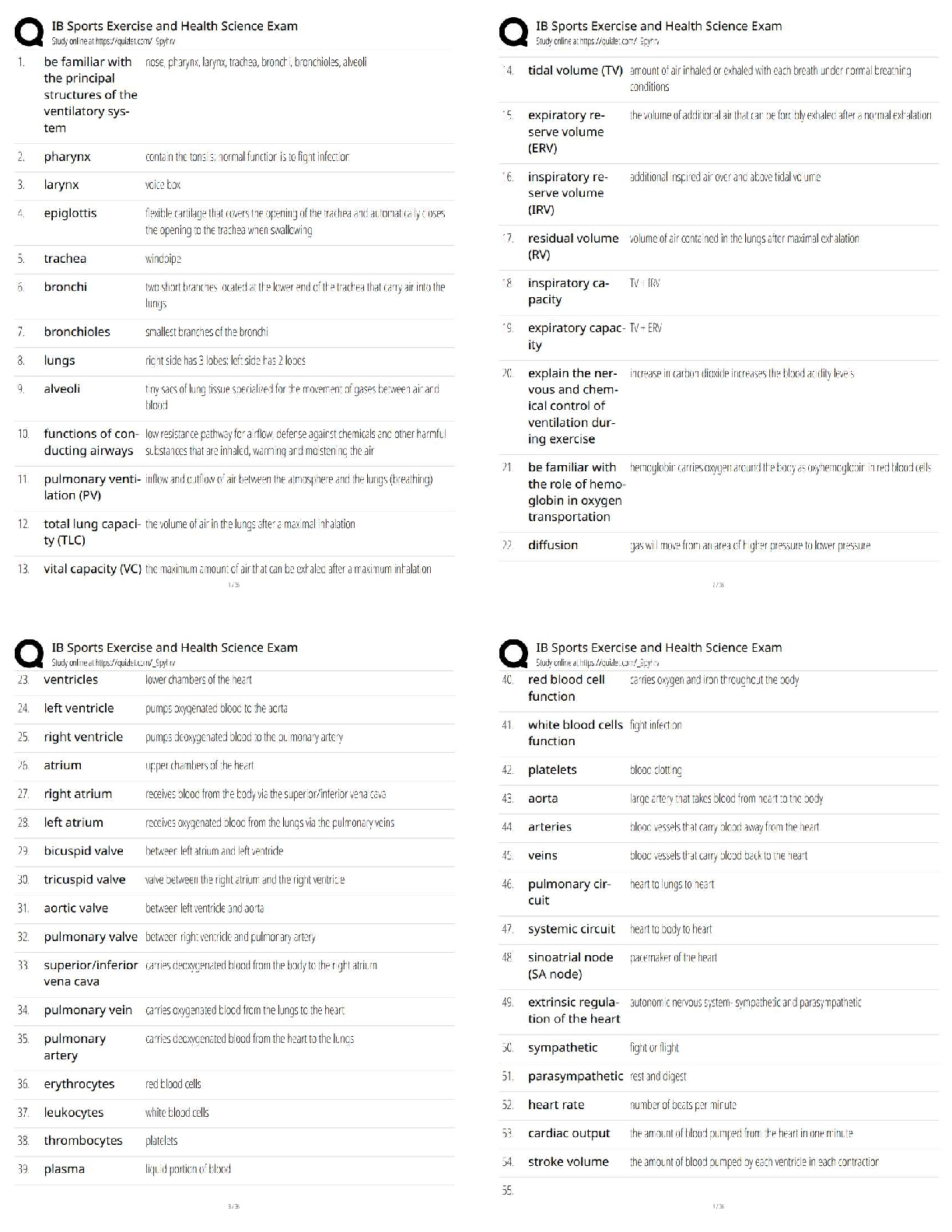
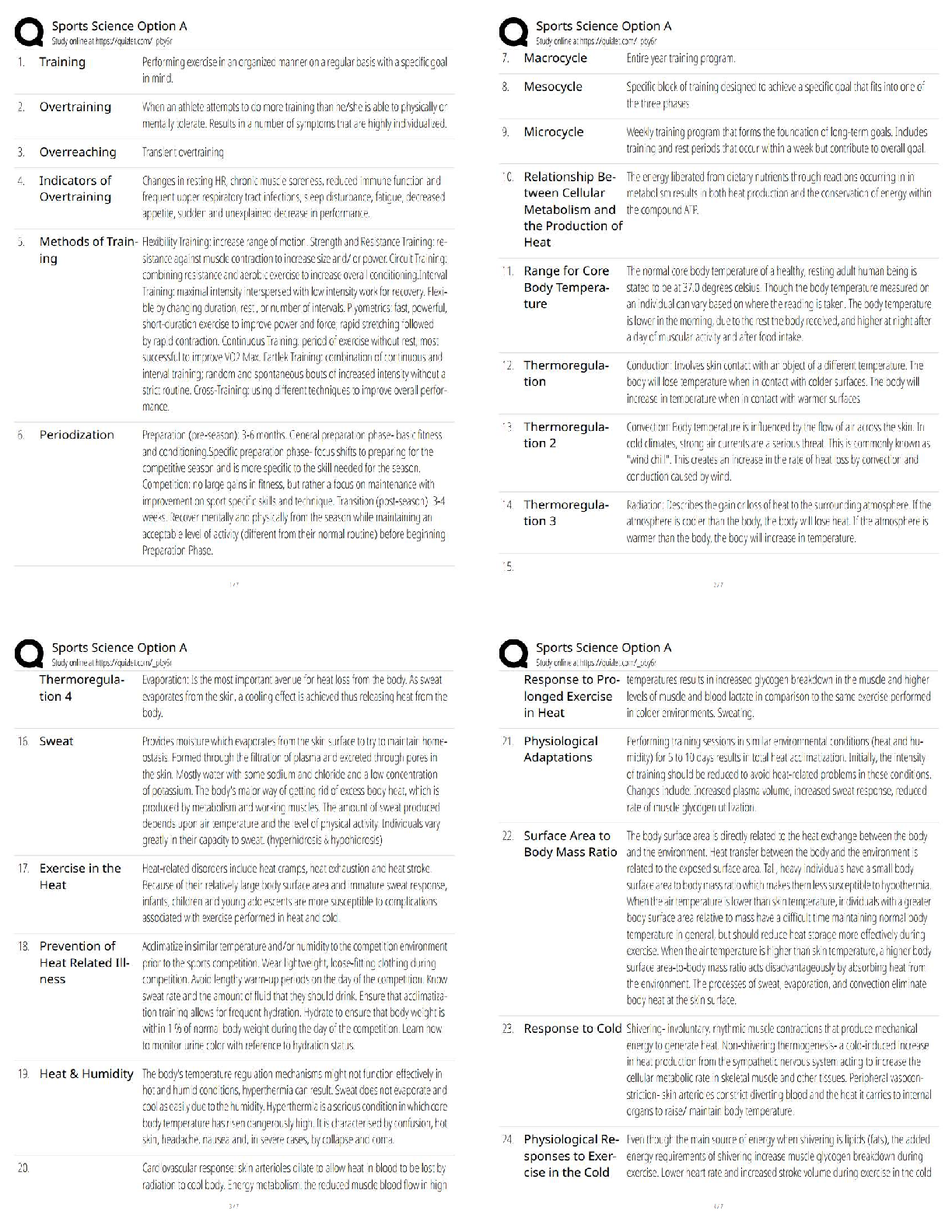
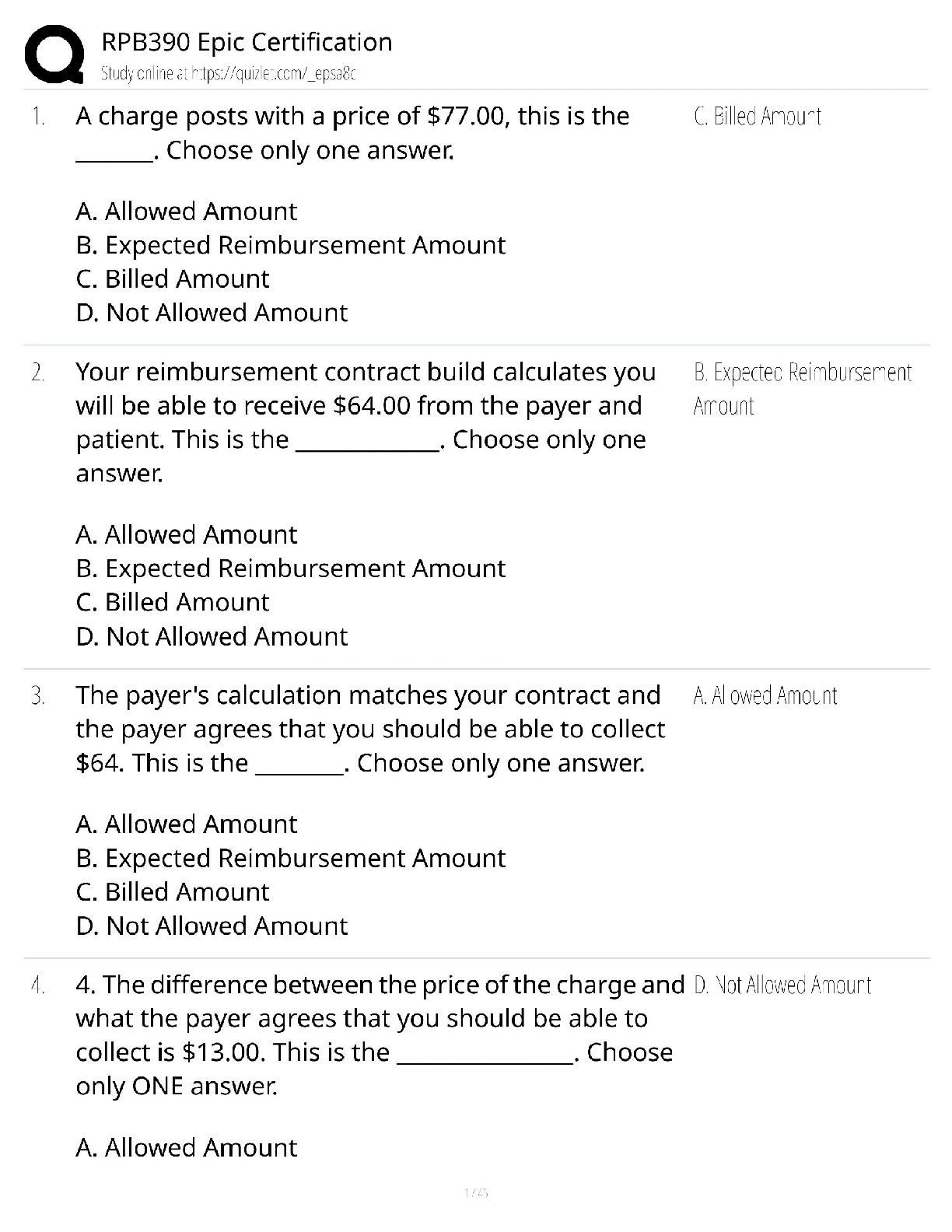
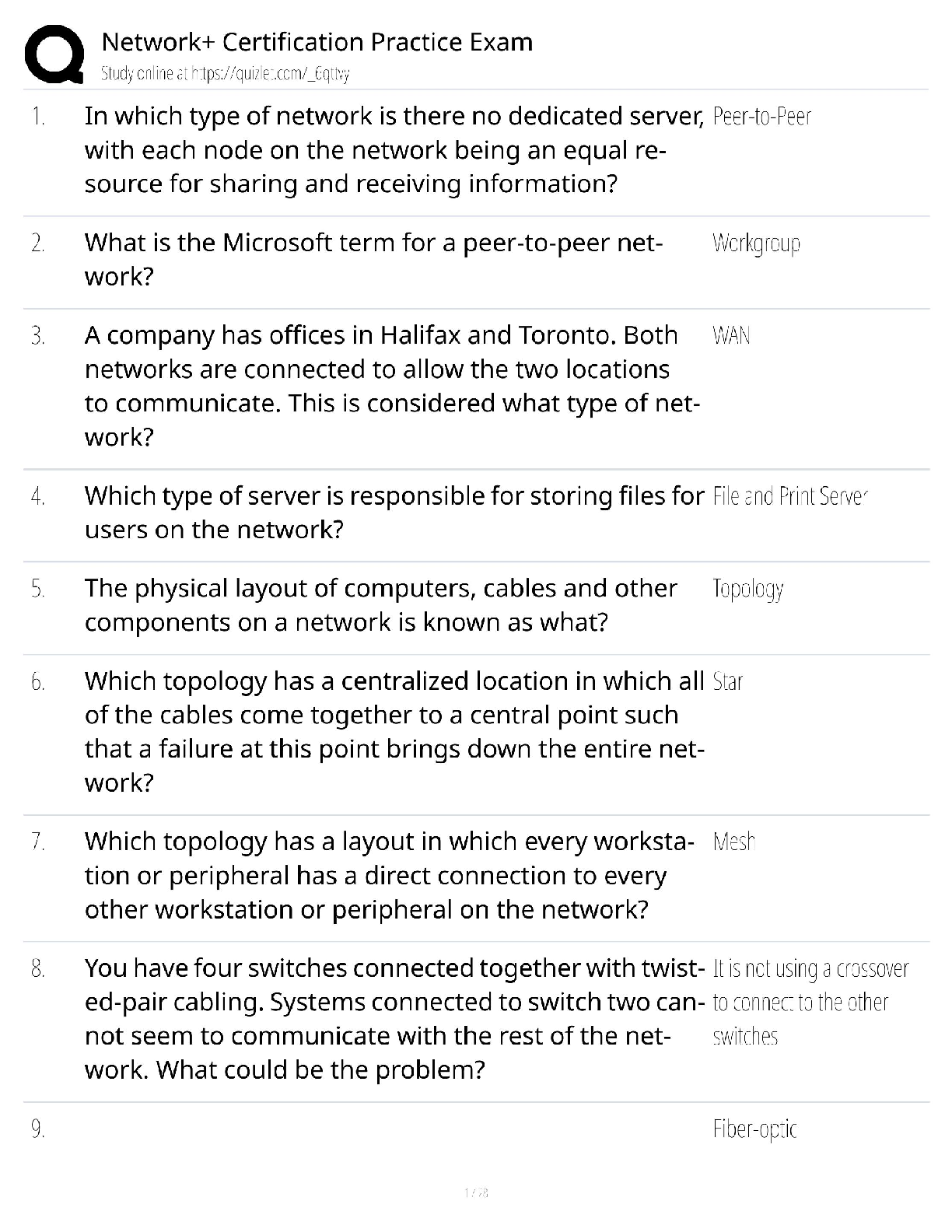
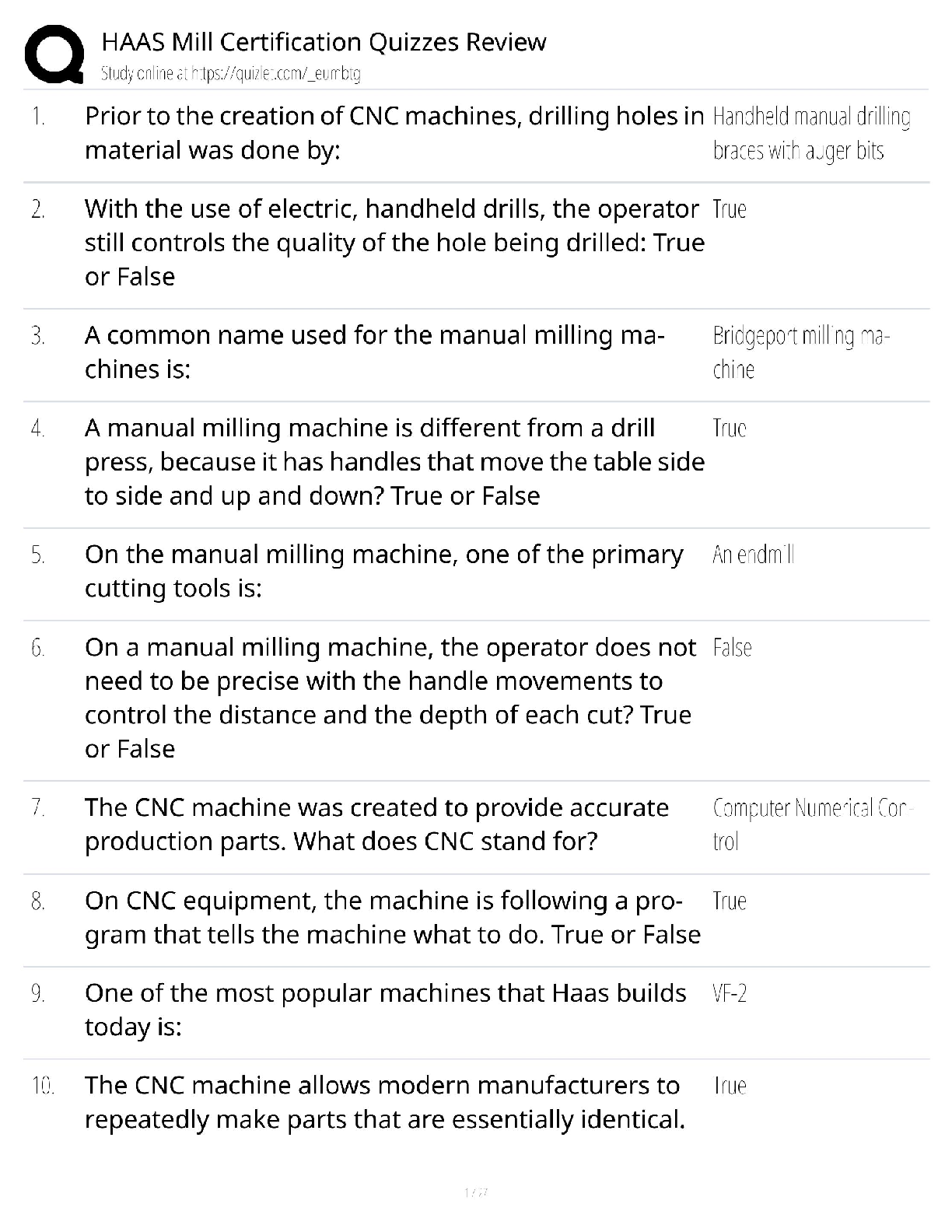
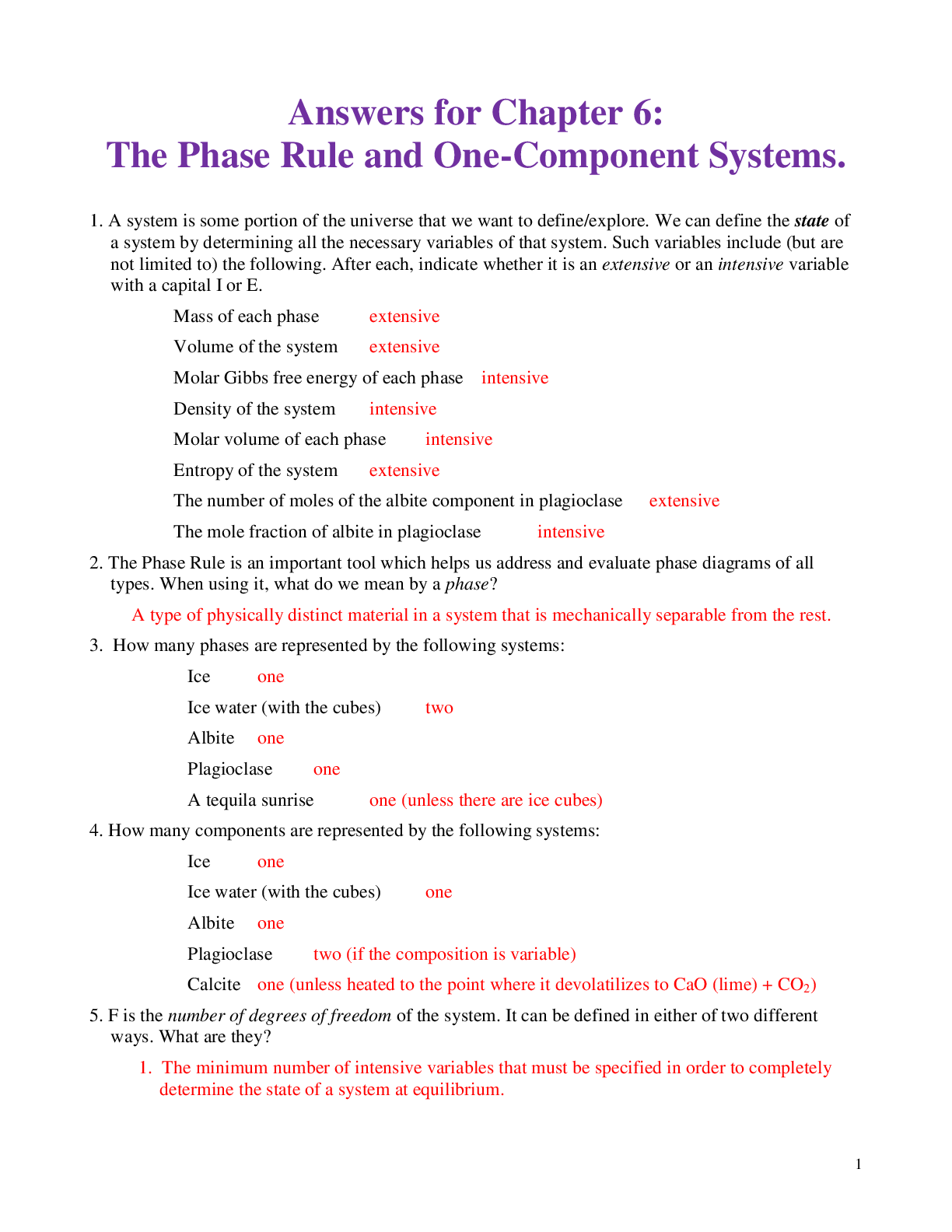
Answers for Chapter 6: The Phase Rule and One-Component Systems. 1. A system is some portion of the universe that we want to define/explore. We can define the state of a system by determining all ... the necessary variables of that system. Such variables include (but are not limited to) the following. After each, indicate whether it is an extensive or an intensive variable with a capital I or E. Mass of each phase Volume of the system Molar Gibbs free energy of each phase Density of the system Molar volume of each phase Entropy of the system The number of moles of the albite component in plagioclase The mole fraction of albite in plagioclase 2. The Phase Rule is an important tool which helps us address and evaluate phase diagrams of all types. When using it, what do we mean by a phase? 3. How many phases are represented by the following systems: Ice one Ice water (with the cubes) two Albite one Plagioclase 4. How many components are represented by the following systems: Ice Ice water (with the cubes) Albite Plagioclase Calcite 5. F is the number of degrees of freedom of the system. It can be defined in either of two different ways. What are they? 6. The diagram at right is a portion of the SiO2 phase diagram (Fig. 6.6). a. Determine F at point x in the diagram. Show your work. What are some possible intensive variables that you could determine? b. Do the same for point y. Compared with point x, are there more or fewer variables that could be determined? Is it necessary to determine more or fewer? Why? c. Assuming that pressure is variable, in a single sentence, describe as accurately and completely as you can the meaning of F in this particular circumstance. I’ll get you started: In a one-component system composed of coexisting coesite and liquid, any change in pressure requires d. What feature on the diagram is a graphic representation of F as it applies to coexisting coesite and liquid? e. Whenever the variables in a system are constrained, a degree of freedom is lost for each one so constrained. Suppose, for example, that a magmatic system cools and crystallizes at a constant pressure (called an “isobaric” system). In what way might we modify Equation 6.1 of the phase rule for such an analysis? f. Assume you heat a system in the lab at a constant pressure with coexisting coesite and liquid (i.e. the furnace is on, consuming energy). Determine F. Does the temperature rise? If so, why? If not, where does the heat go? g. Sketch a graph of temperature vs. time for constant isobaric heating of coesite from 2300oC to 2700oC at 5 GPa. What discontinuities do you note? h. When F is limited, there is generally a reaction taking place. Write the reaction. If pressure is held constant, is the reaction a continuous reaction or a discontinuous reaction? Why? i. Which phase has the larger molar volume, coesite or -quartz? How do you infer this from the phase diagram? . j. Which phase has the larger entropy, coesite or -quartz? How do you infer this from the phase diagram? k. Use the Clapeyron equation (dP/dT = S/V) to determine the sign of the slope of the coesite - -quartz reaction surface. Does your result agree with the diagram? l. Determine F at point z. Describe F for such a situation in words. 7. If you combine 5 grams of NaAlSi3O8 with 3 grams of CaAl2Si2O8 what is the weight fraction of albite in the resulting plagioclase? Show your work. What is the weight fraction of anorthite? Express the plagioclase composition as Anxx (by weight). 8. Plagioclase contains Na, Ca, Al, Si, and O. For phase rule treatment, what is C? Why? 9. The temperature-composition (T-X) Ab-An binary phase diagram at 1 atmosphere pressure is shown on the right. a. If we were to heat pure anorthite at this pressure, what would be the variance (F) when it began to melt? Show your work. What intensive variables specifically addressed by the diagram could vary between the first occurrence of melt and the last bit of anorthite to disappear? What extensive variables on the diagram most obviously change during that interval? b. Begin with composition a at 1550oC. What is the weight fraction of An in the liquid ( ) ? c. Determine the variance for this (isobaric) situation (show your work). List the intensive variables that are relevant to this diagram and could contribute to F. Which in your list, if any, are not independent? d. If the liquid of composition a is isobarically cooled, at what temperature will a solid first begin to form? What will be its composition ( )? e. What are the names of the two curves in the diagram? What is the meaning of each? f. Determine F for this situation, showing your work. Does the number of potential intensive variables increase or decrease when compared to the one-phase situation? Does F correspondingly increase or decrease? Why is this? g. As the system continues to (isobarically) cool, temperature is the only intensive variable to change independently. Because F = 1, all of the other variables must vary in some dependent fashion. What feature on the diagram expresses how , for example, varies with T? h. Use the lever principle to determine the relative weight proportions of plagioclase and liquid at 1400oC. Show your work. i. Place an or and E or I before each of the following variables to indicate whether they increase or decrease and are extensive of intensive as temperature is lowered from 1450oC to 1350oC. ↓ I ↓ I ↓ I T ↓ E Amount of liquid ↑ E Amount of plagioclase j. Some chemical reaction must be taking place across this interval to enable the change you have just described. Because the reaction must occur throughout the interval, the reaction is called a continuous reaction, meaning that it will occur incrementally over an interval (in which F = 1) with no discontinuities in any of the variables. If you begin with liquid of composition A (LiquidA) coexisting with solid of composition B (SolidB) propose an appropriate reaction for a single increment along the reaction path. k. At what specific point on the diagram will the reaction terminate? Explain. l. Determine F for all temperatures below this point. m. Over what temperature range did the liquid crystallize? 10. Using the diagram on the right a. Use colored pencils or different pencil lines to draw the liquid/solid evolution paths for both equilibrium melting and perfect partial melting (each drop of liquid produced is removed) of an olivine of composition Fo20 (weight fraction). How do the paths differ? b. Which of the observations on pages 94-95 do the Ab-An and Fo-Fa systems explain? 11. The temperature-composition (T-X) Diopside (CaMgSi2O6)-An (CaAl2Si2O8) binary phase diagram at 1 atmosphere pressure is shown below. a. Begin with a melt of composition XAn = 80 at 1600oC and derive F (remember it’s isobaric). Suggest likely independent T-X variables for F. b. At what temperature will F change, and what occurs at this point? Derive F and suggest likely independent T-X variables. c. Draw in colored pencil the liquid and solid evolution paths for cooling from the first appearance of a solid to about 1280oC. What is the continuous reaction that occurs and how does it differ from the one in the Ab-An system? d. What occurs at 1274oC? Derive F at this point. e. What happens as the system continues to cool (lose heat) at this temperature? Write the reaction involved and compare it to the reaction you wrote for step (c) above. Is the reaction continuous (occurring progressively over a temperature range) or discontinuous (occurring entirely at a single temperature). Suggest a general rule for deriving such reactions based strictly on the positions of the three points along the isothermal tie-line connecting them. f. What must happen before such an isobaric system can reach a lower temperature? Explain. g. Determine the phases at 1200oC and derive F. Use the lever principle to determine the relative proportions of the two phases. h. Over what temperature interval did the liquid of composition a crystallize? i. Briefly describe the cooling of a liquid of composition An15. In what ways does it differ from the cooling of An80? j. Must every liquid of a composition other than pure Di and pure An reach the eutectic upon cooling? Explain. k. What is the composition of the first liquid derived by melting any mixture of Di and An? Explain. l. How can you know whether Di of An will be consumed first by the eutectic reaction when melting a mixture of Di and An under equilibrium conditions? m. Will fractional crystallization (removing the crystals as they form by sinking and burial or floating) affect the path of liquid evolution during cooling? Explain. n. Describe the process of perfect partial melting (removing each drop of liquid from the system as soon as it forms), beginning with a mixture of 60%An and 40%Di (by weight) until the system is entirely molten. o. In what way(s) do continuous and discontinuous reactions differ? p. Which of the observations on pages 94-95 does the An-Di system explain? 1. Cooling melts crystallize from a liquid to a solid over a range of temperature. 2. Several mineral phases crystallize over this temperature range, and the number of minerals tends to increase as temperature decreases. 3. Minerals usually crystallize sequentially, generally with considerable overlap. 5. The melt composition also changes during crystallization. 6. The minerals that crystallize, as well as the sequence in which they form, depend on the temperature and composition of the melt. 12. The isobaric temperature-composition (T-X) Ab-Or binary phase diagram at 1 atmosphere pressure is shown below. a. Label the field that was left blank. b. Begin with composition Ab40 at 1200oC. Determine the variance for this (isobaric) situation (show your work). List the intensive variables that are relevant to this diagram and could contribute to F. Which in your list, if any, are not independent? c. Draw the liquid and solid evolution curves to 1000oC. Determine F at this temperature and indicate what likely independent intensive T-X variables might be represented. d. Use the lever principle to determine the proportions of liquid and feldspar at 1000oC. Show your work. e. At what temperature will the remaining melt be consumed by the continuous reaction consuming it? Make your reasoning clear. f. ow many phases are present for the Ab40 bulk composition at 900oC? What is the variance (assuming isobaric) and what are the most probable independent intensive T-X variables? Suggest a typical geologic setting for such a situation. g. Describe what would occur under equilibrium conditions as the feldspar in part (f) above were to continue cooling. Draw the equilibrium path(s) on the diagram and determine the variance, suggesting the most probable independent and dependent intensive T-X variables. h. What would be the most probable texture of the cooled feldspar in the situation described immediately above? Why? 13. The isobaric temperature-composition (T-X) Fo-SiO2 binary phase diagram at 1 atmosphere pressure is shown below. i. Begin with composition a at 2000oC. Determine F at this point. j. At what temperature will the first solid form, and what will that solid be? Show your work on the diagram. k. Draw the solid and liquid evolution curves to 1700oC and determine F at this temperature. Suggest the most probable independent and dependent intensive T-X variables under these circumstances. l. What new phase appears at 1557oC? Determine F at this temperature. m. As in the Di-An eutectic situation, there are three phases that plot in a collinear fashion along an isothermal-isobaric tie-line. Use their positions to infer the proper discontinuous reaction relating them under these conditions. What “rule” did you use? How does this reaction differ from the Di+An+Liq eutectic reaction? n. Consider the system in a geologic sense for a moment. You began with a melt in a magma chamber at a constant pressure. As the melt cooled olivine appeared, then orthopyroxene. What happened next? Why? What feature of the Makaopuhi Lava Lake does this explain? o. In the present situation, which of the three phases will be consumed first by the reaction? On what do you base your answer? p. What is the final equilibrium mineral assemblage of the resulting rock (and in what proportions do they occur)? Refer to Chapter 2 and name the rock. q. Suppose the system were 32 wt.% SiO2, rather than 10%. How would the cooling between 1557oC and 1543oC differ from the example just discussed? Explain fully. r. What is the variance and the nature of the continuous reaction between 1557oC and 1543oC? s. What is the variance and the nature of the discontinuous reaction at 1543oC? Which phase must be consumed before the temperature can be lowered further? Why? t. Describe the system and the variance at 1500oC. u. Describe the system and the variance at 1470oC. What is the nature of the discontinuous reaction at this temperature? What exactly is it? v. Describe the melting of pure enstatite until it is entirely molten. w. At equilibrium for an entirely solid mineral assemblage with no solid solution, the isobaric variance should be one, because only temperature can change (there are no compositional variables in the pure solids). If C = 2 and F = 1, then = 2. That is exactly the number of phases in the areas at the bottom of the diagram. If the bulk composition is to the left of the En point, the mineral assemblage must be Fo + En. If the bulk composition is to the right of the En point, the mineral assemblage must be En + Q. Why can’t Fo + Q occur? 14. Describe the cooling history of bulk compositions a, b, and c in Figure 6.7c (below). For each, indicate the temperature at which crystallization begins and ends, and the composition of all phases that form. Describe the nature of any continuous and discontinuous reactions that occur and what is responsible for any changes in . PROBLEMS 1. Illustrate each of the observations listed on pages 94-95 using an example from one of the phase diagrams. 1. Cooling melts crystallize from a liquid to a solid over a range of temperature. 2. Several mineral phases crystallize over this temperature range, and the number of minerals tends to increase as temperature decreases. 3. Minerals usually crystallize sequentially, generally with considerable overlap. 4. Minerals that involve solid solution change composition as cooling progresses. 5. The melt composition also changes during crystallization. 6. The minerals that crystallize, as well as the sequence in which they form, depend on the temperature and composition of the melt. 7. Pressure can affect the temperature range at which a melt crystallizes. It may also affect the minerals that crystallize. . 8. The nature and pressure of any volatile components (such as H2O or CO2) can also affect the temperature range of crystallization and the mineral sequence. 2. Choose a composition on each side of the eutectic minimum of Figure 6.17c and discuss the cooling history of a melt in terms of the phase rule and the intensive variables involved. 3. Binary Phase Diagram Problem Given the 2-component phase diagram above at P = 1 atm. Budlite has the formula AC, and Bovene has the formula BC. The absissa is then 100B/(A+B), which we shall call “mole%B”. Answer the following questions. (2) 1. What is the formula of Wallaclase? (6) 2. Label all fields with appropriate phase(s). 3. Begin with a liquid of composition P. (2) a) Use the phase rule to derive F at 1500oC: (2) b) At what temperature does the liquid begin to crystallize? (2) c) What is the name of the curve that you intersect at this temperature? (3) d) What is the name and mole % B of the phase that forms? (3) e) Use the phase rule to derive F at this point. (5) f) What is the reaction that occurs while there is a liquid and a single solid present during cooling? Is the reaction continuous or discontinuous? Why? Is there a solid reactant? (5) g) What happens when the system just reaches 1000oC? (3) h) Determine F at this temperature. (2) i) What is the name of point x? (2) j) What is the composition of the liquid (in mol % B). (5) k) What is the molar ratio of Budlite to liquid at the instant you reach this temperature (before a new phase forms)? Be accurate and show your work with clear labels and references to the diagram. (5) l) What must happen before you can continue to lower the temperature? Explain using the phase rule. (5) m) What is the reaction that takes place at 1000oC? (3) n) How can you determine what the reaction is strictly from the positions of the phase compositions (5) o) What proportion of the crystallization of the original liquid occurred in the 500oC interval between 1500oC and 1000oC and what proportion took place at 1000oC? Be specific. (hint: use your answer to k) (4) p) Assuming equilibrium crystallization, What is the ratio of Budlite to Wallaclase (in moles) in the final rock? (2) q) What effect would fractional crystallization have on the path the liquid follows? (3) r) What effect would fractional crystallization have on the composition of the final rock? Compare the mineral percentages of the final equilibrium crystallization rock and that of the fractionally crystallized rock. (2) 4. a) If a liquid of composition Q is cooled from 1500oC, at what temperature will it begin to crystallize? (2) b) At what temperature will it become completely solid? (2) c) Explain your answers to the two previous questions. An eutectic composition crystallizes at a single temperature, because Bl and W are present in just the right ratio. (3) 5. a) If pure Wallaclase is heated, at what temperature will it melt? b) What will be the product(s) of this melting, and what is this type of melting behavior called? (3) (2) c) What is the composition of the liquid (in mol % B) (2) d) What is point y called? (5) e) If you begin with 10 moles of Wallaclase, how much liquid can be generated at this temperature? Show your work. (2) f) At what temperature will melting be complete? 6. If a rock of composition R, containing Budlite and Wallaclase is heated. (2) a) At what temperature will it begin to melt? (1) b) What will be the composition (in mol %B) of the first melt to form? (2) c) What must happen before the temperature can be raised further? Why? (4) d) How can you use the tie-line geometry to determine which phase will be the first to be consumed? (2) e) At what temperature will the last solid phase disappear? (1) f) What solid is it? 7. Cool a magma of composition S. (2) a) At what temperature will the first crystals appear? (2) b) What mineral is it? (3) c). At what temperature during cooling will F change next, and why? Be specific. (4) d) At this temperature can you use the lever principle to determine the relative proportions of liquid and solid that are present in the system? Why or why not? Because L + Bv W, the amounts of all 3 phases will change. (3) e) What must happen before you can lower the temperature further? Use the phase rule to show your reasoning. (5) f) How can you use the positions of the compositions of the phases and the bulk composition along the tie-line to determine which phase will have to disappear in this situation? L (5) g) Write the reaction occurring. Is it continuous or discontinuous? Why? (2) h) What will be the composition (in mol % B) of the last drop of liquid to crystallize? (4) i) What will it crystallize to, and at what temperature? (4) 8. a) If a magma with a bulk composition A:B ratio of 1:4 (be careful here) is cooled from 1800oC, at what temperature will it first begin to crystallize? (2) b) At what temperature will it become completely solid? (5) c) What will be mineralogy and ratio of the minerals in the final rock? (5) d) If fractional crystallization were to occur until 1200oC, and equilibrium crystallization after that, what would be the mineralogy and mineral ratio in the final rock? (5) 9. In what rocks would you find coexisting Budlite and Bovene at equilibrium? Explain. (8) 10. If you began with a rock containing approximately equal amounts of Wallaclase and Bovene, describe the simplest geologically plausible method by which you would end up with a mixture of Wallaclase and Budlite. [Show More]
Last updated: 3 years ago
Preview 1 out of 20 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$13.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Feb 08, 2021
Number of pages
20
Written in
All
Additional information
This document has been written for:
Uploaded
Feb 08, 2021
Downloads
0
Views
145