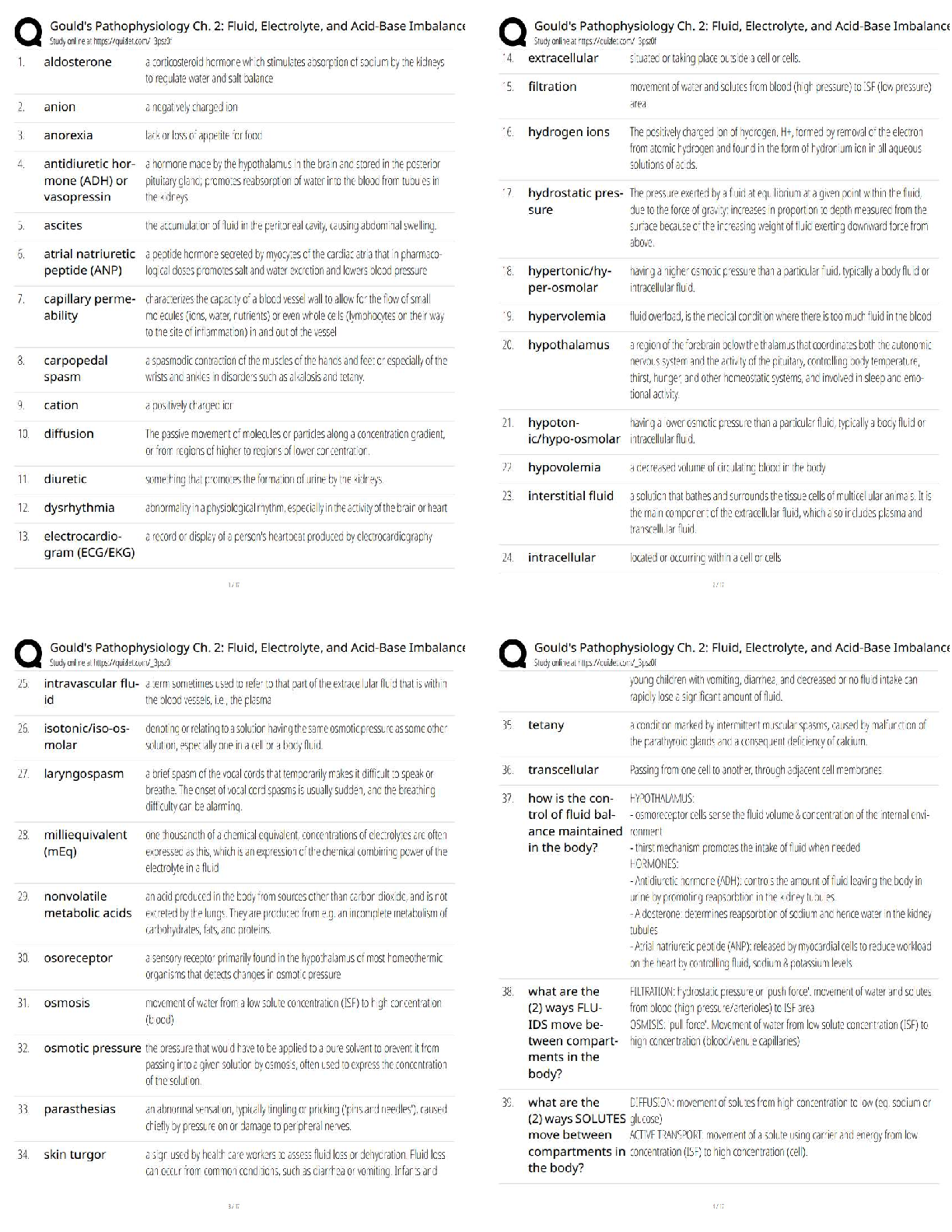
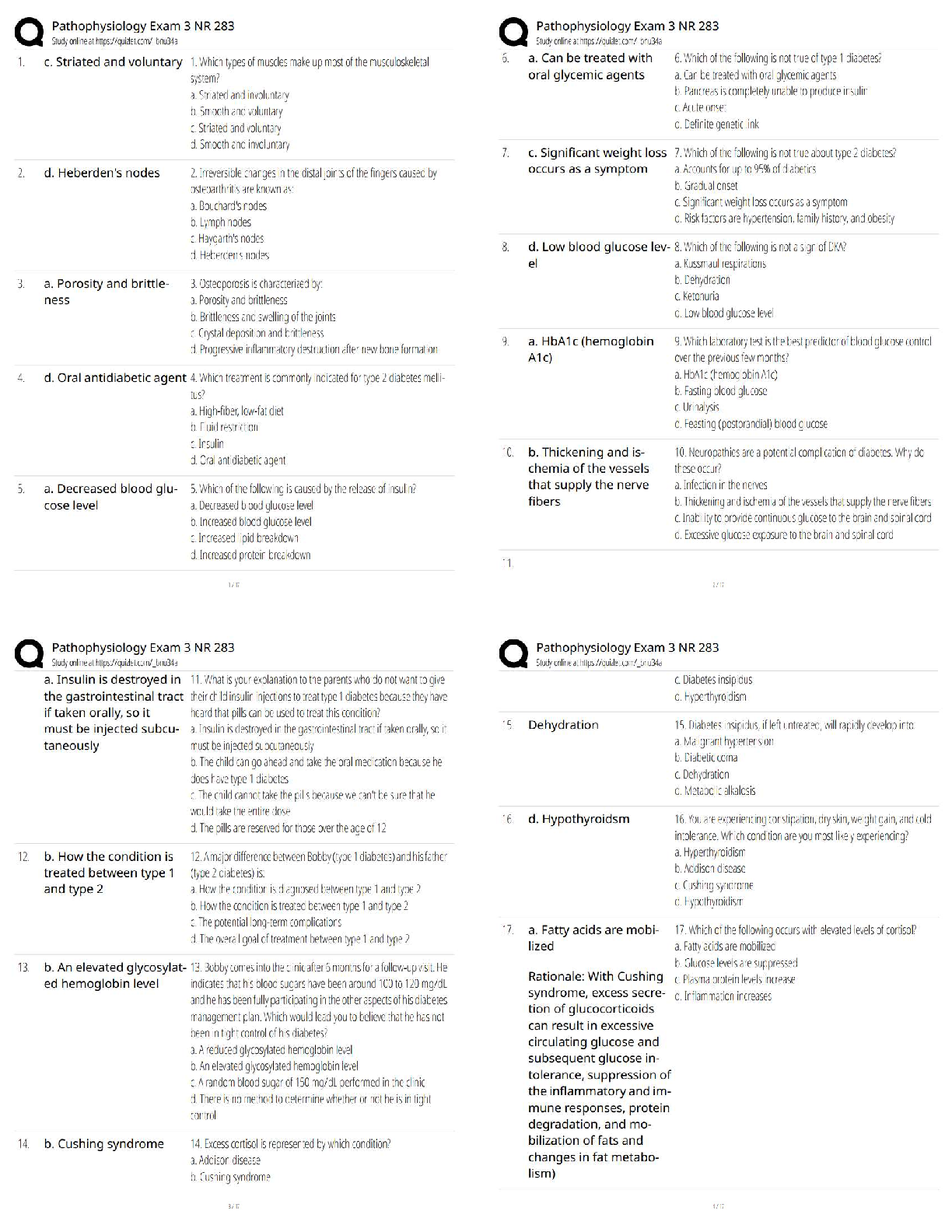
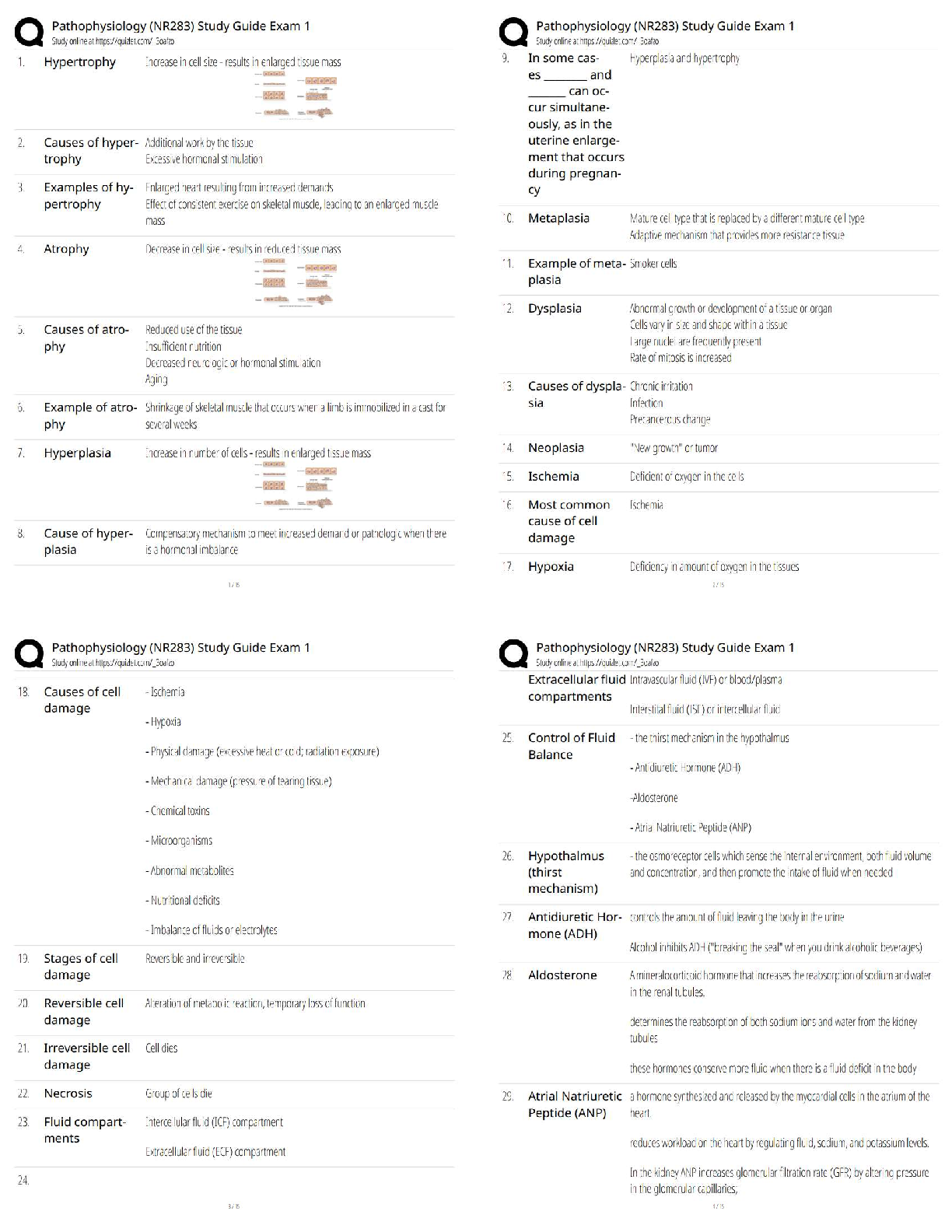
Topic 2: Chemistry for Anatomy and Physiology Students
• BIO201_MH_V3
• Topic 2: Chemistry for Anatomy and Physiology Students
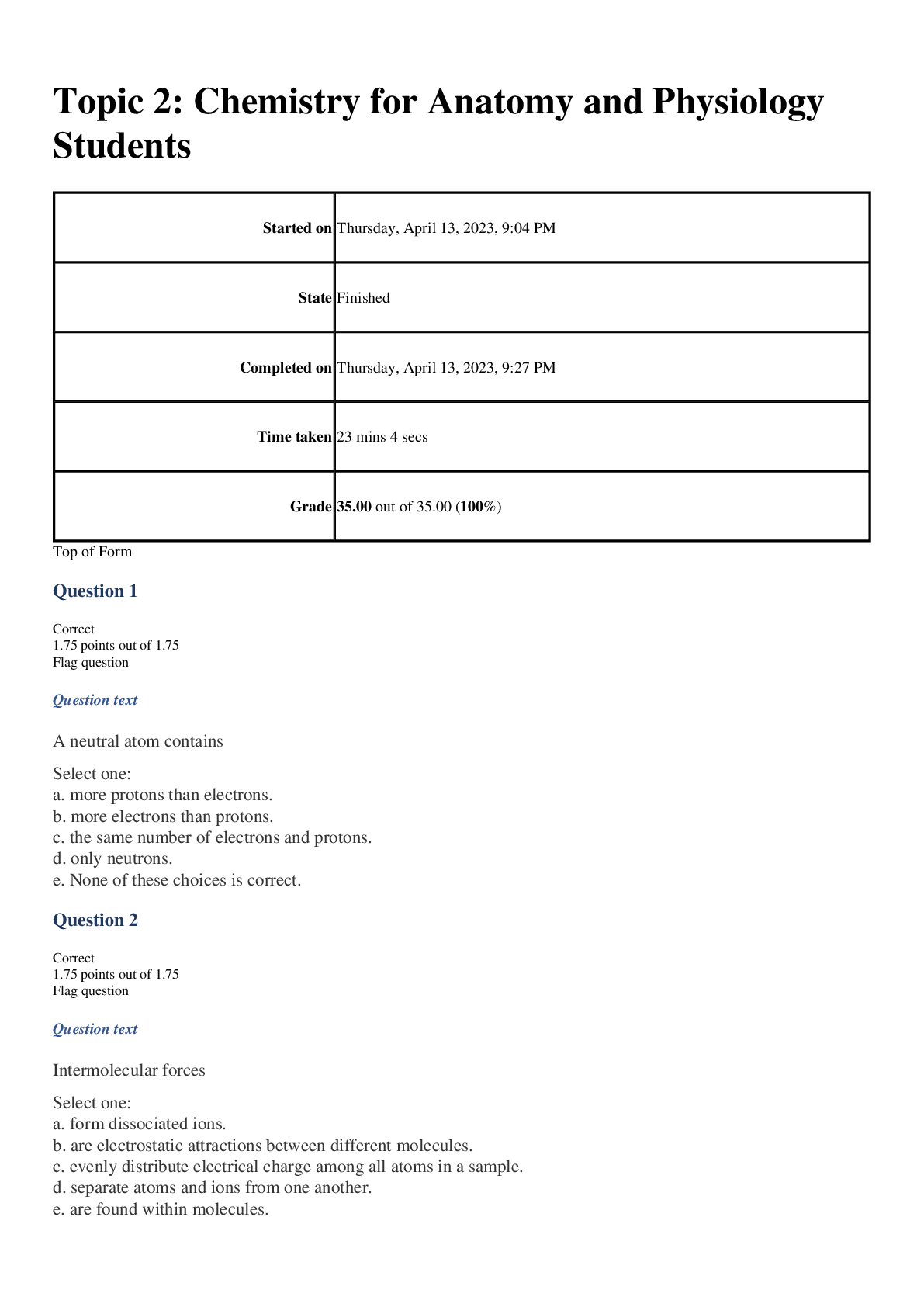
Started on Thursday, April 13, 2023, 9:04 PM
State Finished
Completed on Thursday, April
...
Topic 2: Chemistry for Anatomy and Physiology Students
• BIO201_MH_V3
• Topic 2: Chemistry for Anatomy and Physiology Students
Started on Thursday, April 13, 2023, 9:04 PM
State Finished
Completed on Thursday, April 13, 2023, 9:27 PM
Time taken 23 mins 4 secs
Grade 35.00 out of 35.00 (100%)
Question 1
Correct
1.75 points out of 1.75
Flag question
Question text
A neutral atom contains
Select one:
a. more protons than electrons.
b. more electrons than protons.
c. the same number of electrons and protons.
d. only neutrons.
e. None of these choices is correct.
Question 2
Correct
1.75 points out of 1.75
Flag question
Question text
Intermolecular forces
Select one:
a. form dissociated ions.
b. are electrostatic attractions between different molecules.
c. evenly distribute electrical charge among all atoms in a sample.
d. separate atoms and ions from one another.
e. are found within molecules.
Question 3
Correct
1.75 points out of 1.75
Flag question
Question text
The number of atoms in exactly 12 grams of carbon-12 is called
Select one:
a. Dalton's number.
b. Socrates's number.
c. Avogadro's number.
d. Pasteur's number.
e. Le Chatelier's number.
Question 4
Correct
1.75 points out of 1.75
Flag question
Question text
Electrolytes are substances that
Select one:
a. form covalent bonds with water.
b. conduct electricity when dissolved in water.
c. cannot conduct electricity in solution.
d. are NOT found in the human body in any appreciable amounts.
e. are NOT charged particles.
Question 5
Correct
1.75 points out of 1.75
Flag question
Question text
Subatomic particles located around the nucleus of an atom are
Select one:
a. protons.
b. electrons.
c. neutrons.
d. neutrinos.
e. photons.
Question 6
Correct
1.75 points out of 1.75
Flag question
Question text
The smallest particle of an element that still exhibits the chemical characteristics of that element is a(n)
Select one:
a. electron.
b. atom.
c. chemical bond.
d. orbital.
e. proton.
Question 7
Correct
1.75 points out of 1.75
Flag question
Question text
Electrons
Select one:
a. comprise the majority of the mass of an atom.
b. are located in the nucleus of an atom.
c. have a positive charge of one.
d. are the subatomic particles most involved in bonding behavior of atoms.
e. do not participate in the bonding of atoms.
Question 8
Correct
1.75 points out of 1.75
Flag question
Question text
The four most abundant elements in the human body are
Select one:
a. carbon, hydrogen, oxygen, and iron.
b. carbon, hydrogen, oxygen, and nitrogen.
c. calcium, hydrogen, sodium, and potassium.
d. carbon, oxygen, magnesium, and zinc.
e. carbon, sulfur, calcium, and potassium.
Question 9
Correct
1.75 points out of 1.75
Flag question
Question text
When ionic compounds dissolve in water, their ions
Select one:
a. cling tightly together.
b. dissociate or separate from one another.
c. lose their charge.
d. get lost in the solvent.
e. settle to the bottom of the container.
Question 10
Correct
1.75 points out of 1.75
Flag question
Question text
Which of the following pairs is mismatched?
Select one:
a. synthesis reaction - two reactants combine to form a larger product
b. decomposition reaction - large reactant broken into smaller products
c. oxidation - gain of electrons
d. dehydration reaction - water is a product of the reaction
e. hydrolysis - water is used in decomposition reaction
Question 11
Correct
1.75 points out of 1.75
[Show More]