Exploring Strategy 11th edition Chapter 1-16 STUDY GUIDE
$ 20
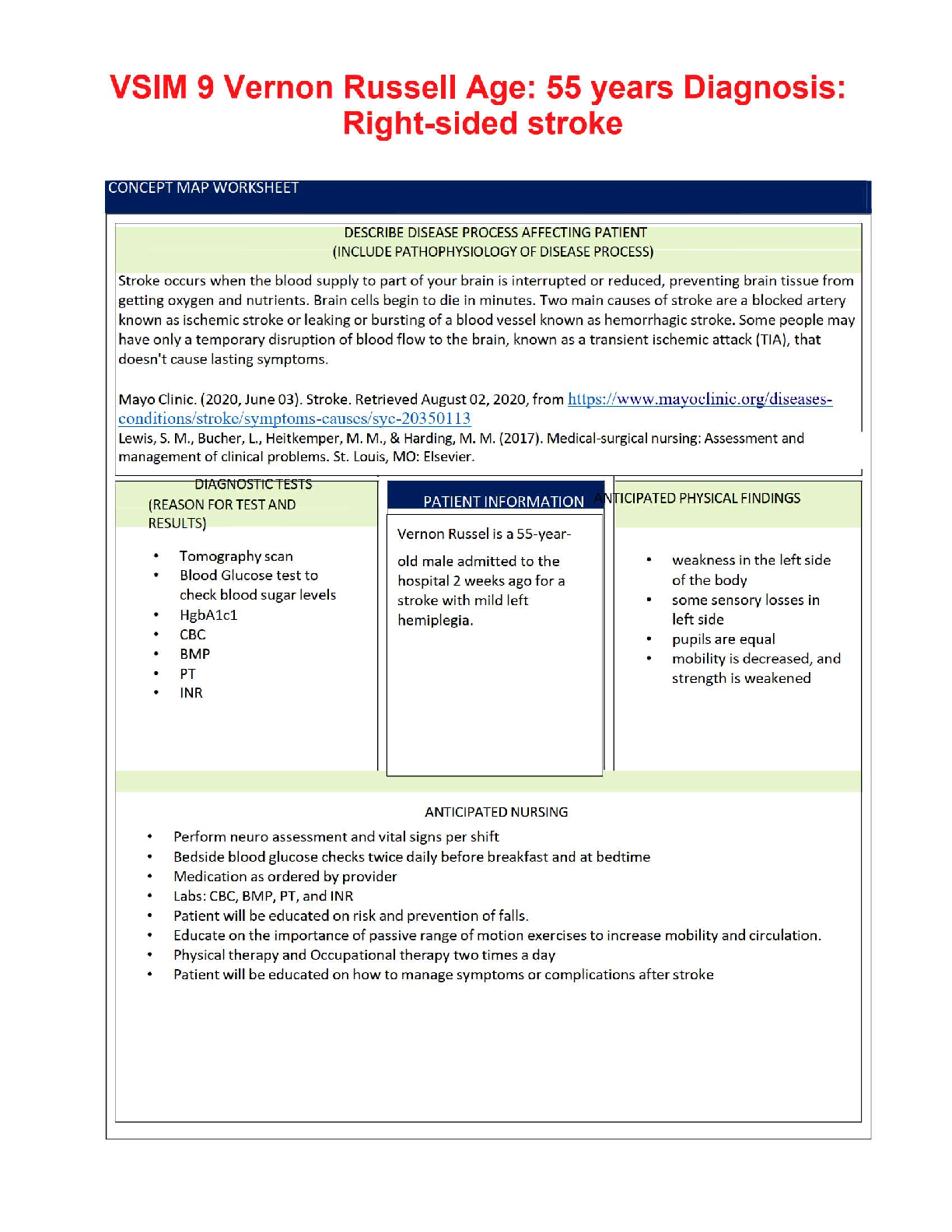
📄 VSIM 9: Vernon Russell – Right-Sided Stroke Case Study
$ 14.5

ATI Maternal Newborn Practice B
$ 15

The Great Gatsby Test Multiple Choice Study Choice/ Latest Update
$ 11.5

Canadian Advertising in Action, 11e Keith J. Tuckwell (Solution Manual)
$ 25

eBook Bioenhancement and Fortification of Foods for a Healthy Diet 1st Edition By Octavio Paredes-López ,Oleksandr Shevchenko , Viktor Stabnikov , Volodymyr Ivanov
$ 30

TEST BANK FOR UNDERSTANDING PATHOPHYSIOLOGY 7TH EDITION BY HUETHER
$ 20
.png)
HESI EXIT RN EXAM 2022 - REAL RATED A+
$ 24

GFEBS Spending Chain Purchase Orders Brand NEW | Latest 2023-2024 | Complete Questions & Answers (Solved)