TCIC/LETS Full Access with CCH/CCQ Re-Certification Test Latest Update/ Download
$ 10

ATI MATERNAL NEWBORN PROCTORED 2019
$ 10

NURS 629 Peds Exam 2 QUESTIONS WITH COMPLETE SOLUTIONS
$ 12

Excel Crash Course Exam from Wall Street Prep - Wall Street Prep
$ 7

Professional Nursing II Exam 2 |PN2 Exam 2; Complete Solution, Already Graded A
$ 12

NGN ATI PN COMPREHENSIVE PREDICTOR EXAM TEST BANK A+ GRADED
$ 48.5

IICRC ISSI Comprehensive Test Guide 2024
$ 13

IICRC RFI Comprehensive Test Guide 2024
$ 13

NURS 6512N -Week 4 Shadow Health Tina Jones Transcript.
$ 12

ATI COMPREHENSIVE EXIT EXAM 2023 WITH NGN
$ 13
.png)
Dosage calculation practice problems questions and answers 100% pass
$ 7.5

ECO-561-Final-Exam-7 2023 Microeconomics
$ 11

CPCU 500 Exam Study Guide
$ 15
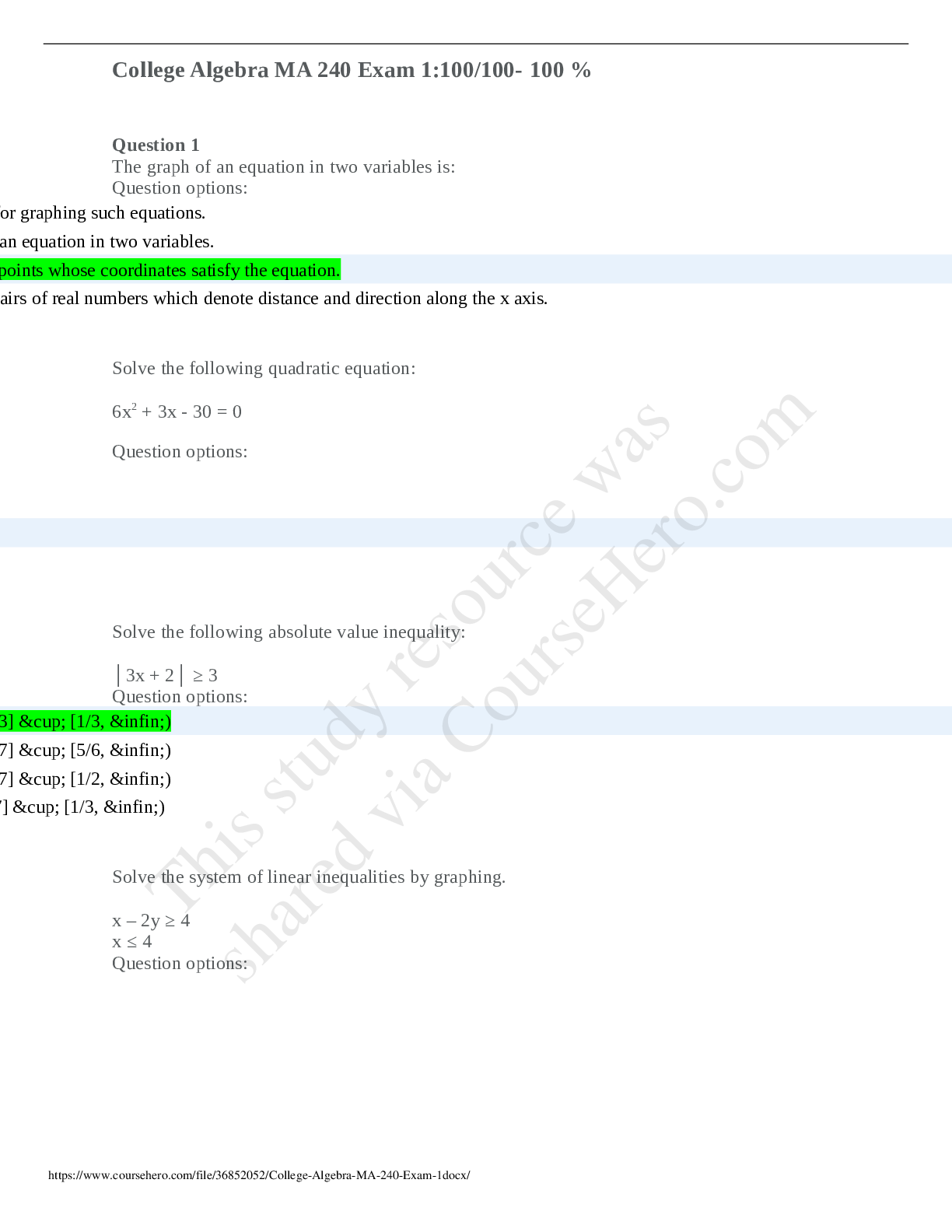
College Algebra MA 240 Exam
$ 12.5
 Correct Study Guide, Download to Score A.png)
Case Study Appendicitis Appendectomy, SKINNY Reasoning, Suggested Answer Guidelines, John Washington, 14 years old, (Latest 2021) Correct Study Guide, Download to Score A
$ 18

HSM 542 Week 4 Midterm Exam with Answers
$ 20
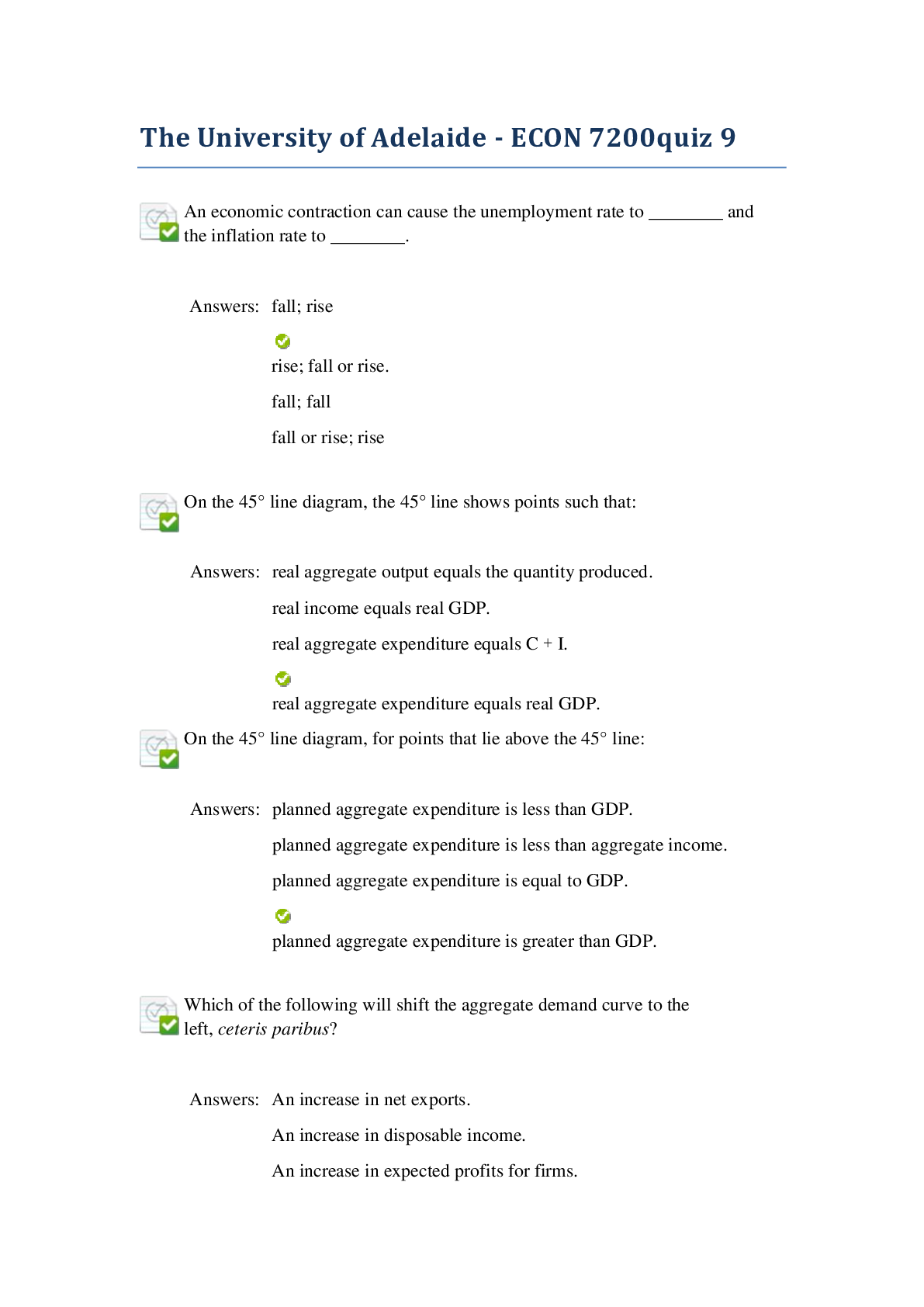
The University of Adelaide - ECON 7200quiz 9 . Graded A and Reviewed
$ 9

Elementary Principles of Chemical Processes 4th Edition by Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
$ 16

A-level MATHEMATICS 7357/2 Paper 2 Mark scheme June 2022
$ 9

HUMAN BIOLOGY UNIT 2 MILESTONE | GUARANTEED PASS. Already Graded A+
$ 8.5

ATI Community Health Proctored Exam
$ 12

NUR 307 Pharm Final Exam 1 (120 Q&A),100% CORRECT
$ 18

Univaersity of Arizona: ECON 332: SPRING 2020 Final Exam. Scored 97%
$ 17

PHARM NR 508 Advanced Pharmacology QUIZZES
$ 64

FINAL for Role and Scope/Leadership