Chemistry > ASSIGNMENT > CHEM120 Week 2 Assignment: Bonding and Naming of Molecules – With 100% Correct Answers Download To (All)
CHEM120 Week 2 Assignment: Bonding and Naming of Molecules – With 100% Correct Answers Download To Score An A+
Document Content and Description Below
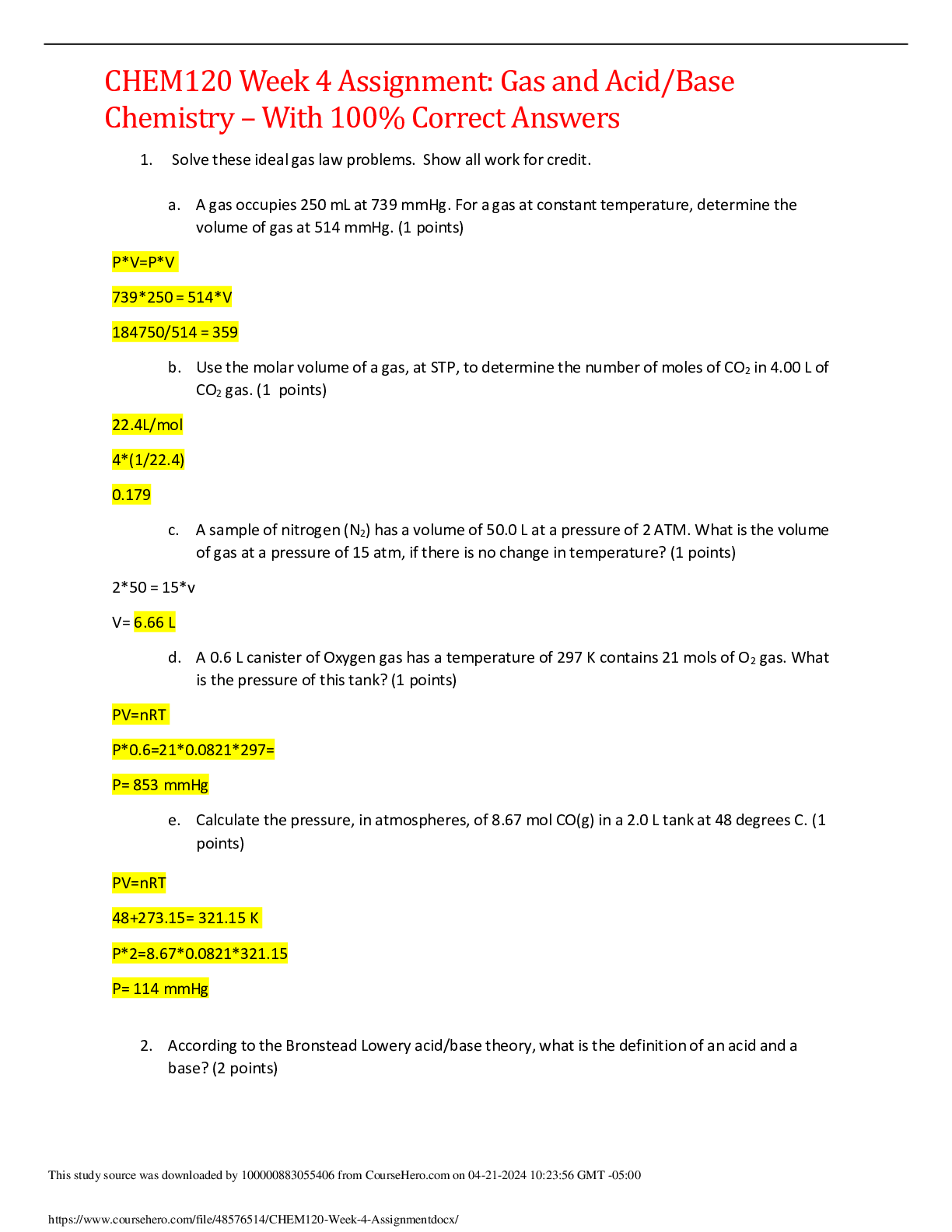
CHEM120 Week 2 Assignment: Bonding and Naming of Molecules – With 100% Correct Answers Download To Score An A+ Week 2 Assignment (15 points): 1. In terms of electrons, what is an excited sta ... te? Give an example of an electron configuration for an excited state. (1 points) - An electron is in an excited state when it occupies an energy state that’s greater than its ground state. The ground state of Oxygen for example has an electron configuration that is 1s22s22p4 but the excited state might show as 1s22s22p33s1 where the valence electron now occupies the 3s orbit. 2. Give the ground state electron configuration for Fe. (1 points) - The ground state of iron is 1s22s22p63s23p63d6 3. What is a valance electron? How many valance electrons does Si have? (1 points) - A valence electron is a single electron or one of two or more electrons in the outermost shell of an atom. It’s responsible for the chemical properties of the atom. Si has 4 valence electrons. 4. What is the shorthand electron configuration of a potassium ion (K+)? (1 points) - [Ar] 4s1 5. Give the chemical formula (with charge) for each of the following polyatomic ions: (0.5 points each, 2.5 points total) a. Phosphate – O4P-3 b. Ammonium – NH + c. Sulfate – SO42- d. Hydroxide – OH- e. Carbonate – CO3-2 6. What is an Ion and what is the difference between an anion and a cation? (1 points) - An ion is an atom with a net electrical charge - A cation is when an atom loses one or more electrons and has a positive charge - A anion is when an atom gains one or more electrons and has a negative charge 7. What is the difference between Ionic bonds and covalent bonds? (1 points) - In covalent bonds, atoms share electrons, are formed between two non-metals, and the reaction is electrically neutral. In ionic bonds atoms transfer electrons, the reaction of both atoms are charged, and they are formed between a metal and non-metal. 8. Fill in this table (2.5 points, 0.5 points for each row) Molecule Name Molecular Formula Ionic or Covalent Compound Ferric Oxide Fe2O3 Ionic Magnesium Hydroxide Mg(OH)2 Ionic Chlorine Heptoxide Cl2O7 Covalent Dihydrogen Sulfide H2S Covalent Ammonium Phosphate (NH4)3PO4 Ionic 9. Label the following compounds as containing polar covalent bonds or nonpolar covalent bonds. (0.5 points each, 2 points total) a. O2 : Nonpolar b. SF2 - : Polar c. CH4 : Nonpolar d. Br2 : Nonpolar 10. Draw the electron dot structure for PF3. What is the name of this molecule? (1 points) a. Phosphorus Triflouride 11. What are the molecular geometries of CH4 and SiO2? (0.5 points each, 1 point total) - CH4 : Tetrahedral shape - SiO2 : Tetrahedral shape [Show More]
Last updated: 1 year ago
Preview 1 out of 3 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$13.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 19, 2024
Number of pages
3
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 19, 2024
Downloads
0
Views
80