Chemistry > ASSIGNMENT > CHEM120 Week 4 Assignment: Gas and Acid/Base Chemistry |100% Correct Answers (All)
CHEM120 Week 4 Assignment: Gas and Acid/Base Chemistry |100% Correct Answers
Document Content and Description Below
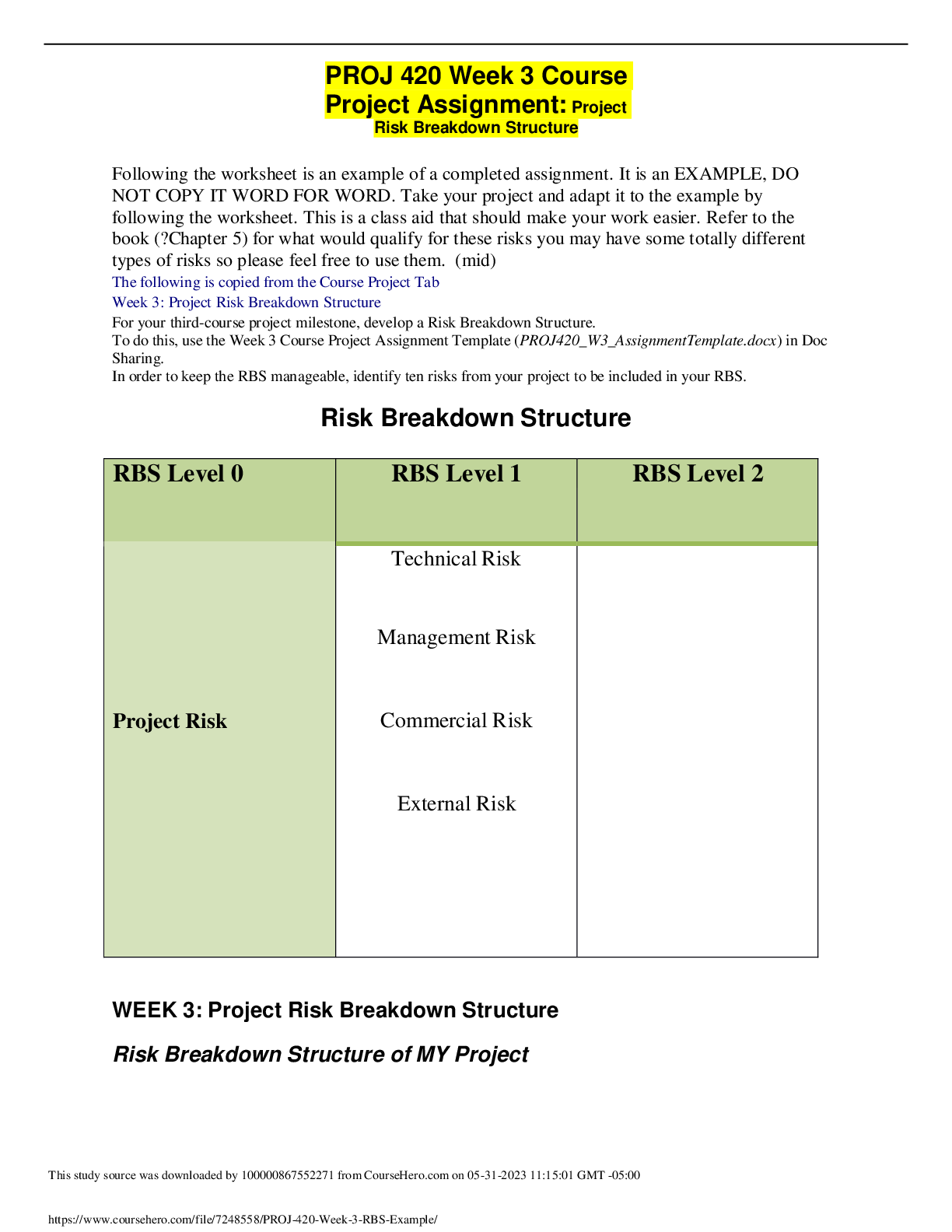
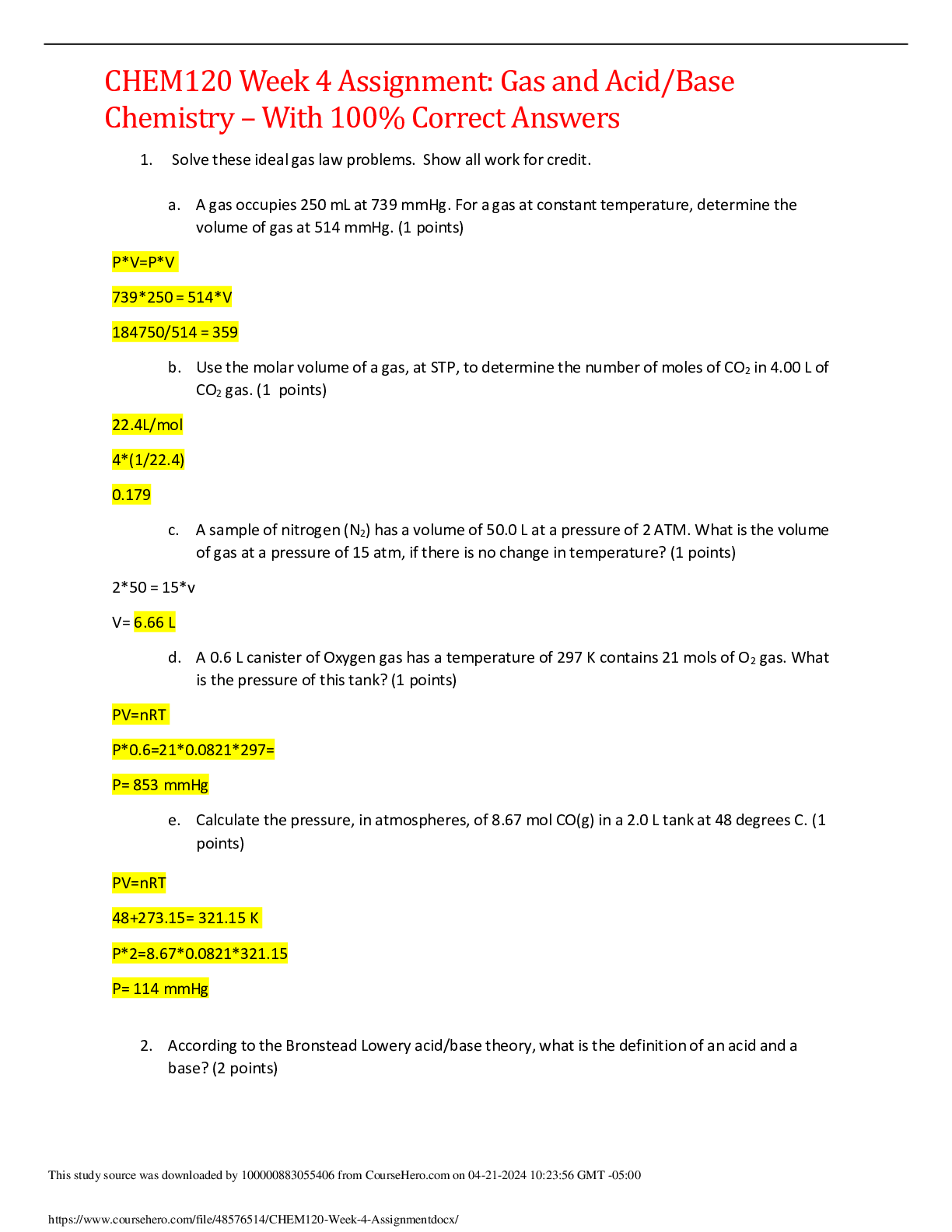
CHEM120 Week 4 Assignment: Gas and Acid/Base Chemistry – With 100% Correct Answers 1. Solve these ideal gas law problems. Show all work for credit. a. A gas occupies 250 mL at 739 mmHg. For a ga ... s at constant temperature, determine the volume of gas at 514 mmHg. (1 points) P*V=P*V 739*250 = 514*V 184750/514 = 359 b. Use the molar volume of a gas, at STP, to determine the number of moles of CO2 in 4.00 L of CO2 gas. (1 points) 22.4L/mol 4*(1/22.4) 0.179 c. A sample of nitrogen (N2) has a volume of 50.0 L at a pressure of 2 ATM. What is the volume of gas at a pressure of 15 atm, if there is no change in temperature? (1 points) 2*50 = 15*v V= 6.66 L d. A 0.6 L canister of Oxygen gas has a temperature of 297 K contains 21 mols of O2 gas. What is the pressure of this tank? (1 points) PV=nRT P*0.6=21*0.0821*297= P= 853 mmHg e. Calculate the pressure, in atmospheres, of 8.67 mol CO(g) in a 2.0 L tank at 48 degrees C. (1 points) PV=nRT 48+273.15= 321.15 K P*2=8.67*0.0821*321.15 P= 114 mmHg 2. According to the Bronstead Lowery acid/base theory, what is the definition of an acid and a base? (2 points) If something gives up an H it is an acid, if something gives up an OH it is a base. If something accepts an H it is a conjugate base and something accepts an OH it is a conjugate acid. 3. According to the Bronstead Lowery acid/base theory, determine the acids and bases in the following chemical reactions: (1 point each) HCl + NaOH ⟶ NaCl + H2O Acid base NH + + H O ⇌ H O+ + NH Acid base H2SO4 + NH3 ⟶ NH + + H1SO - Acid base 4. Name the following compounds and identify each of the following as a salt, strong acid, weak acid, strong base, or weak base. (0.5 points each, 3 points total) a. HCl strong acid b. KBr salt c. NH3 weak base d. LiOH strong base e. CH3COOH weak acid f. Ca(OH)2 weak base 5. Rank the following solutions in order of how acidic they are from most acidic to least acidic. (2 points) Solution A: [H3O+] of 1 x 10-2 M Solution B: pH 5 Solution C: [H3O+] of 1 x 10-11 M Solution D: [H3O+] of 1 x 10-7 M Solution E: pH 9 A, B, D, E, C 6. Predict the Products of the reaction below: (1 points) HCl + Ca(OH)2 H2O + CaCl 7. For the following redox reactions, determine what is oxidized and what is reduced: (1 points each, 2 points total) 3 Hg2+ + 2 Fe (s) 3 Hg2 + 2 Fe3+ Hg was reduced, Fe was oxidized 2 As (s) + 3 Cl2 (g) 2 AsCl3 As was oxidized, Cl was reduced 8. State the properties of a buffer solution and the key components of such a solution. (2 points) A solution that resists changes in pH. Often times they have a weak acid and base along with their salt. [Show More]
Last updated: 1 year ago
Preview 1 out of 3 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 22, 2024
Number of pages
3
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 22, 2024
Downloads
0
Views
129