Chemistry > QUESTIONS and ANSWERS > Chem 103 Lab exam 6 Questions and Answers (100% Correct Elaborations) Portage Learning (All)
Chem 103 Lab exam 6 Questions and Answers (100% Correct Elaborations) Portage Learning
Document Content and Description Below
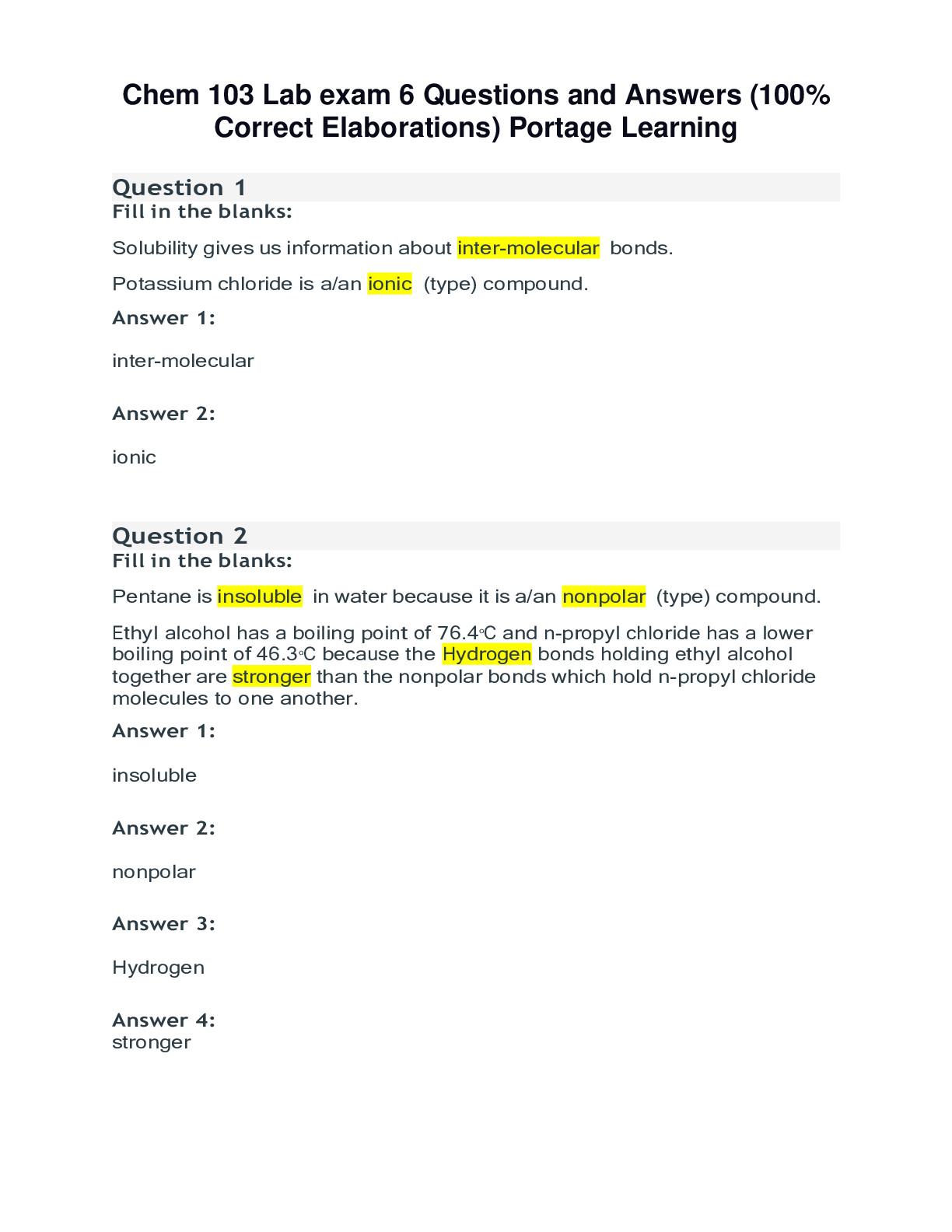
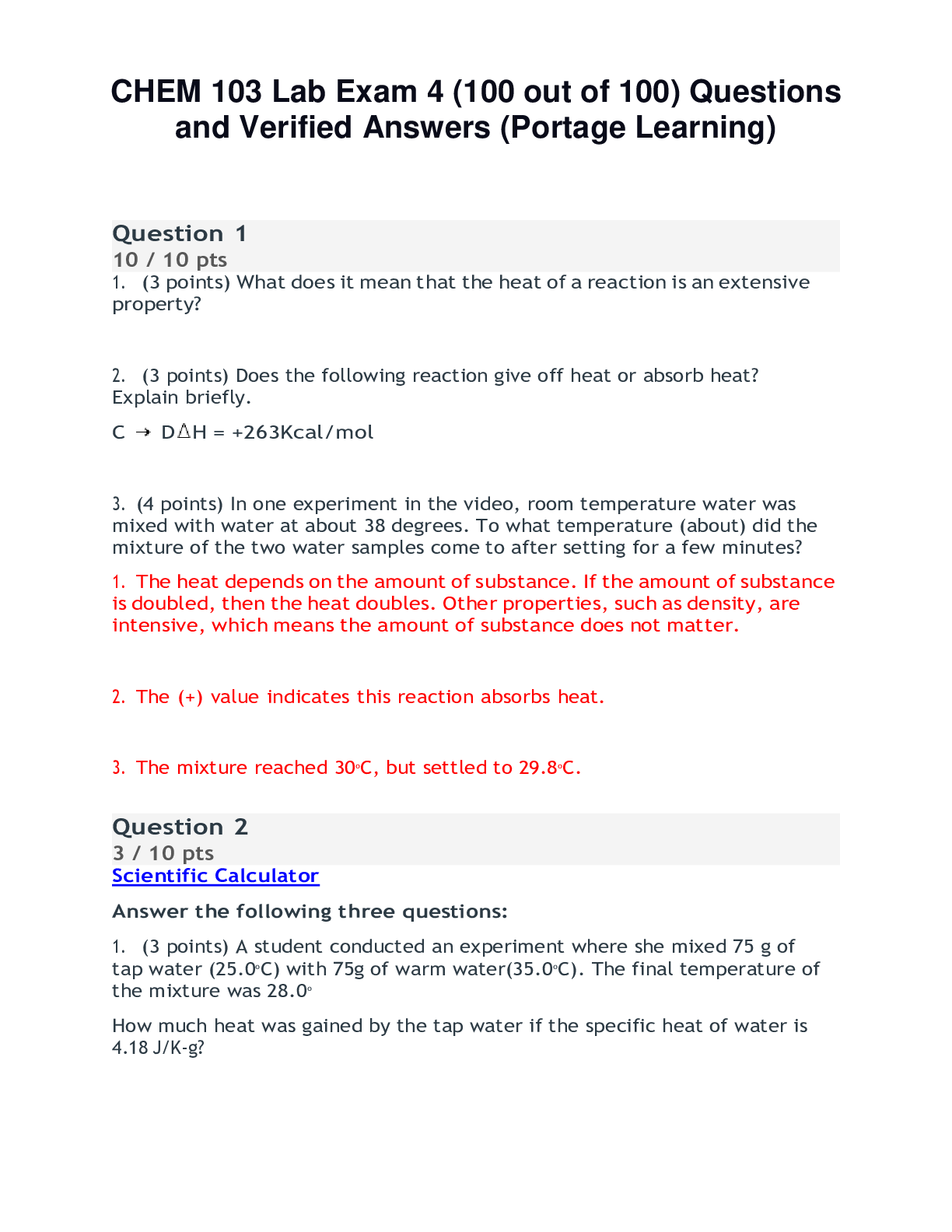
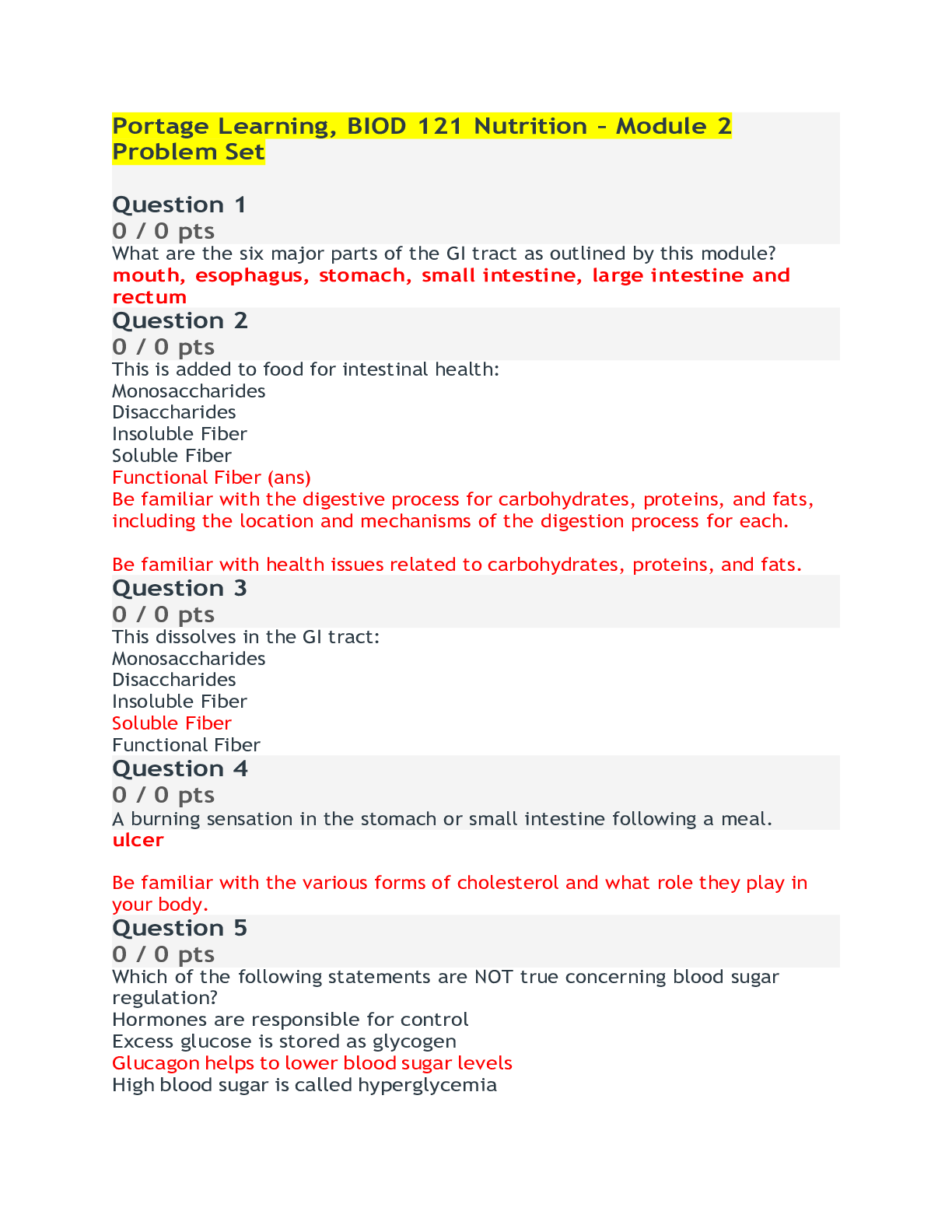
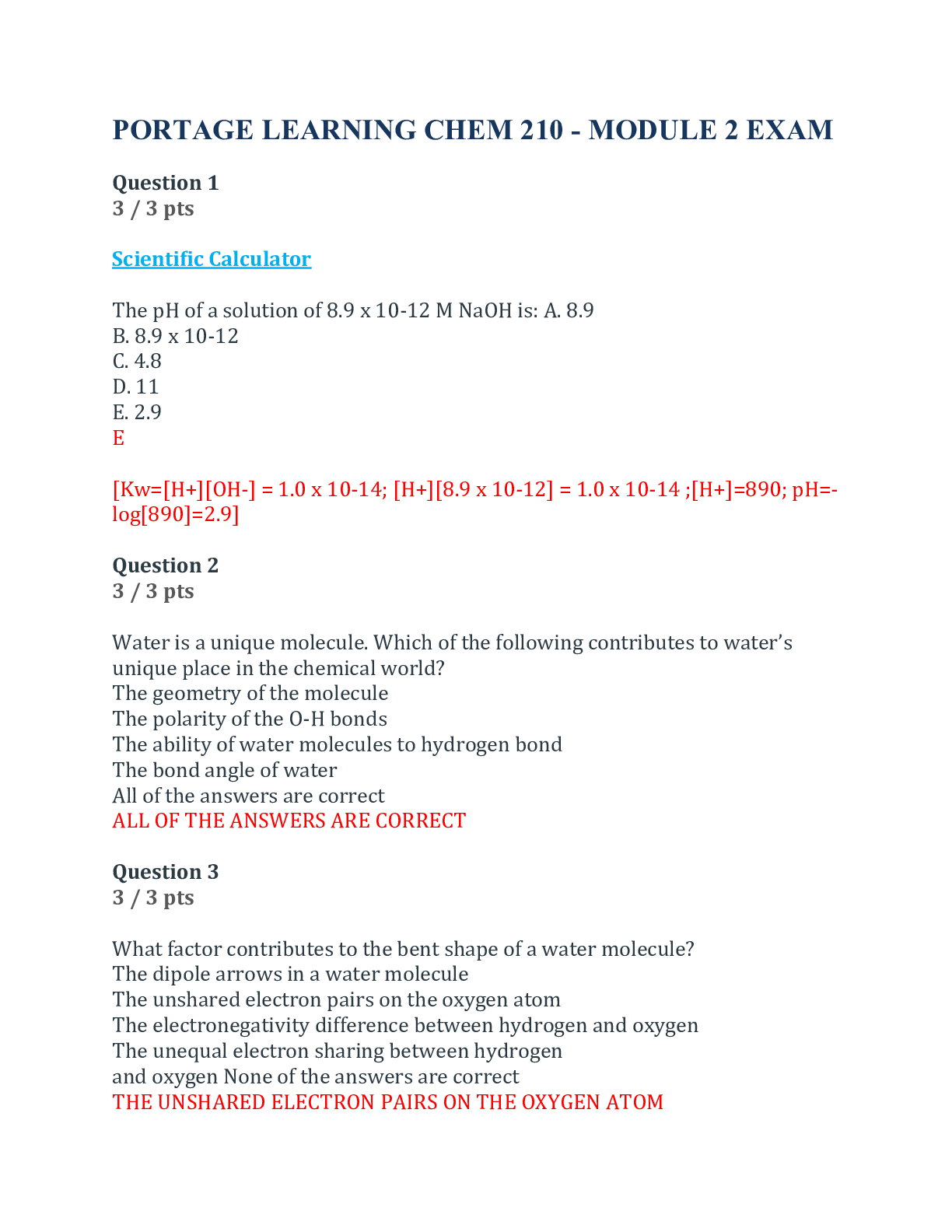
Chem 103 Lab exam 6 Questions and Answers (100% Correct Elaborations) Portage Learning Question 1 Fill in the blanks: Solubility gives us information about inter-molecular bonds. Potassium chlori... de is a/an ionic (type) compound. Answer 1: inter-molecular Answer 2: ionic Question 2 Fill in the blanks: Pentane is insoluble in water because it is a/an nonpolar (type) compound. Ethyl alcohol has a boiling point of 76.4oC and n-propyl chloride has a lower boiling point of 46.3 oC because the Hydrogen bonds holding ethyl alcohol together are stronger than the nonpolar bonds which hold n-propyl chloride molecules to one another. Answer 1: insoluble Answer 2: nonpolar Answer 3: Hydrogen Answer 4: strong [Show More]
Last updated: 8 months ago
Preview 1 out of 11 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$8.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 02, 2024
Number of pages
11
Written in
Additional information
This document has been written for:
Uploaded
Dec 02, 2024
Downloads
0
Views
71


















 – Miami Dade College.png)
Perop.png)


