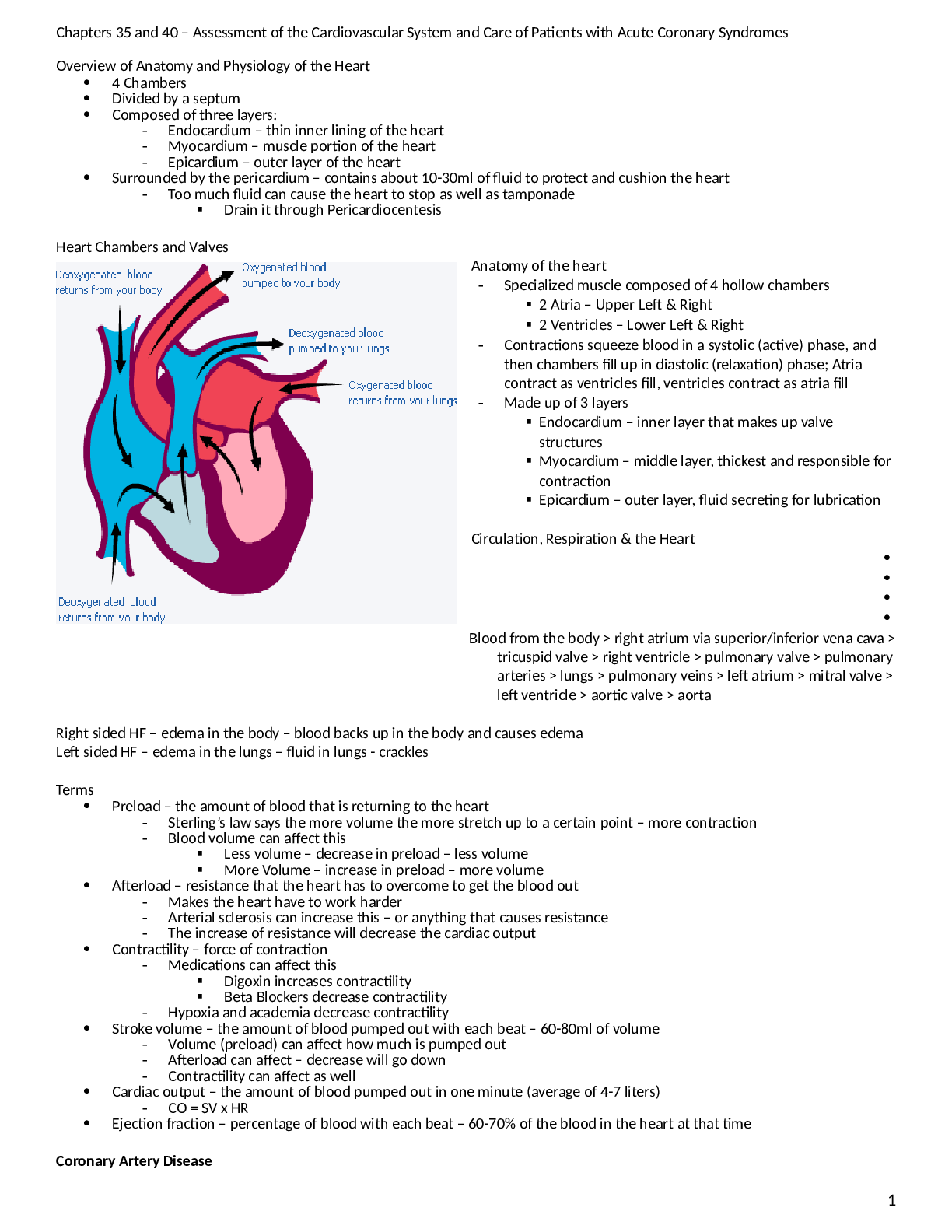
*NURSING > ATI MEDICAL SURGICAL > ATI Medical Surgical Nursing, Assessment and Care of Patients with Acid-Base Imbalances (All)
ATI Medical Surgical Nursing, Assessment and Care of Patients with Acid-Base Imbalances
Document Content and Description Below
In a client with less than the normal amount of bicarbonate in the blood and other extracellular fluids, what response does the nurse anticipate? a. Increased risk for acidosis b. Decreased risk... for acidosis c. Increased risk for alkalosis d. Decreased risk for alkalosis ANS: A Bicarbonate (H2CO3–) is a weak base with an overall negative charge. When hydrogen ions are present in slight or mild excess (mild acidosis), bicarbonate can buffer or absorb the excess hydrogen ions, reducing the hydrogen ion concentration and bringing the pH back up to normal. If the total body bicarbonate concentration is low, especially in the blood, the action of buffering or absorbing excess hydrogen ions is reduced, and the person is at increased risk for acidosis. DIF: Cognitive Level: Comprehension/Understanding REF: p. 202 TOP: Client Needs Category: Physiological Integrity (Physiological Adaptation—Pathophysiology) MSC: Integrated Process: Nursing Process (Assessment) [Show More]
Last updated: 4 months ago
Preview 5 out of 29 pages

Loading document previews ...
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 02, 2025
Number of pages
29
Written in
Additional information
This document has been written for:
Uploaded
Mar 02, 2025
Downloads
0
Views
36