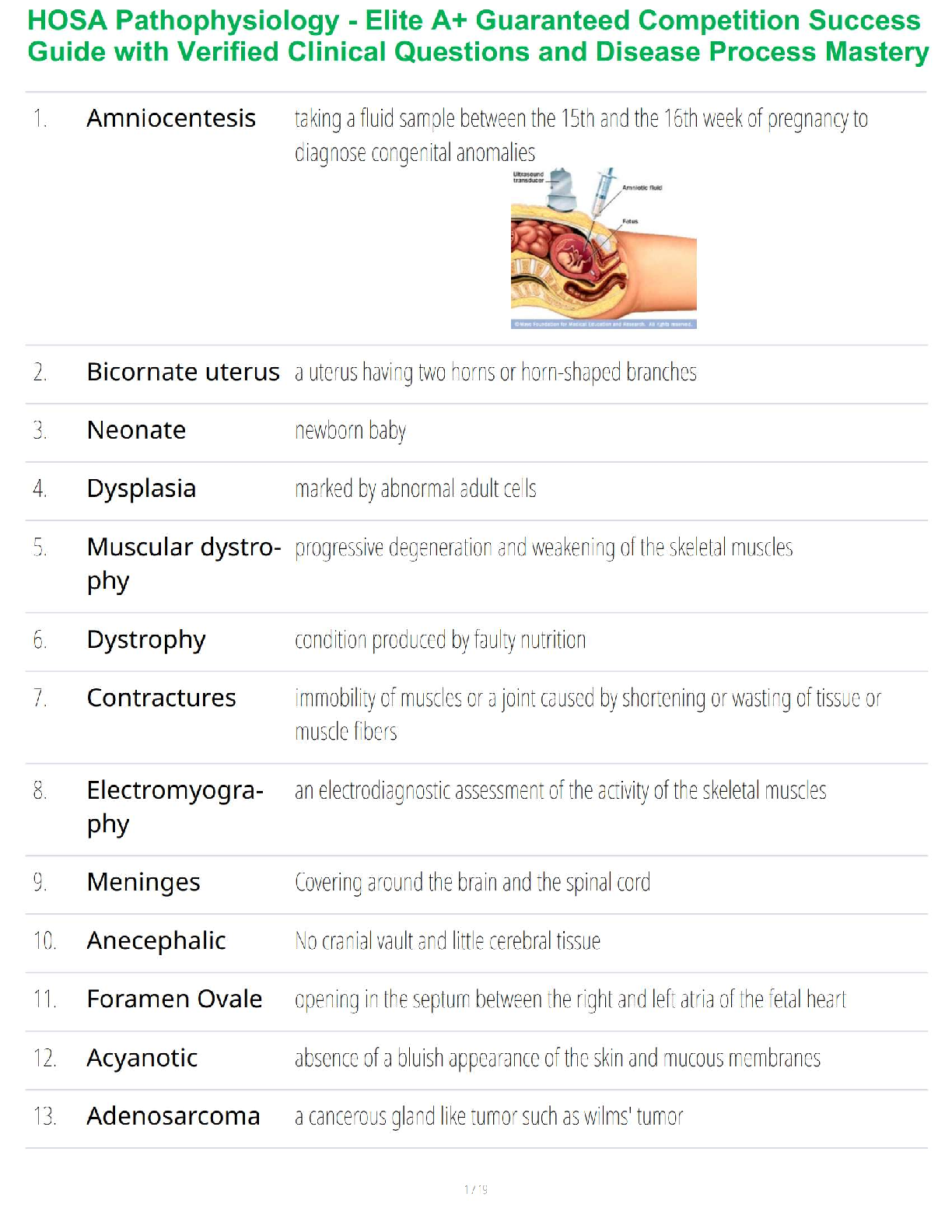
Water/Buffers
• Properties of Water:
o Water is a solvent and is polar (like dissolves like)
Polar due to oxygen being more electronegative than hydrogen
Also undergoes hydrogen bonding between molecular for inte
...
Water/Buffers
• Properties of Water:
o Water is a solvent and is polar (like dissolves like)
Polar due to oxygen being more electronegative than hydrogen
Also undergoes hydrogen bonding between molecular for intermolecular interactions as well as London-dispersion and Van der waals forces
• Hydrogen bonding is present between molecules bonding with hydrogen that have O, N, or F. (note no Carbon)
• These bonds also allow ice to have a lower density than liquid water
The ability of a molecule to form hydrogen bonds with water defines its solubility in water, and the inability to form hydrogen bonds with water is the basis of hydrophobic interactions.
Polar compounds dissolve in water by forming new H bonds with the solute and the process overall being a dynamic process (constant breaking and formation of H bonds.)
Water also dissolves salts extremely well via ion-dipole interactions
• Increase in entropy when a salt is dissolved in water is called Hydration.
Non-polar substances have a different story
• Non-polar substances due to lack of hydrogen bonding and very little difference in electronegativities.
• In water, move in order to minimize the amount of surface exposed to water which is entropically driven.
• Water forms a cage around the non polar solute which leads to a decrease in entropy for water.
• Form chains around the hydrophobic areas
• Hydrophobic interactions – ‘forces’ that hold the non polar regions of the molecules
[Show More]










.png)