Vocabulary: carbohydrate, chemical formula, dehydration synthesis, disaccharide, glucose,
hydrolysis, monosaccharide, polysaccharide, valence
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
1. If you exe
...
Vocabulary: carbohydrate, chemical formula, dehydration synthesis, disaccharide, glucose,
hydrolysis, monosaccharide, polysaccharide, valence
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
1. If you exercise on a hot day, you need to worry about dehydration. In this context, what do
you think dehydration means? A lack of water needed in the body
2. Astronauts and backpackers often bring dehydrated food. What do you think dehydrated
food is? Food without water or moisture
Gizmo Warm-up
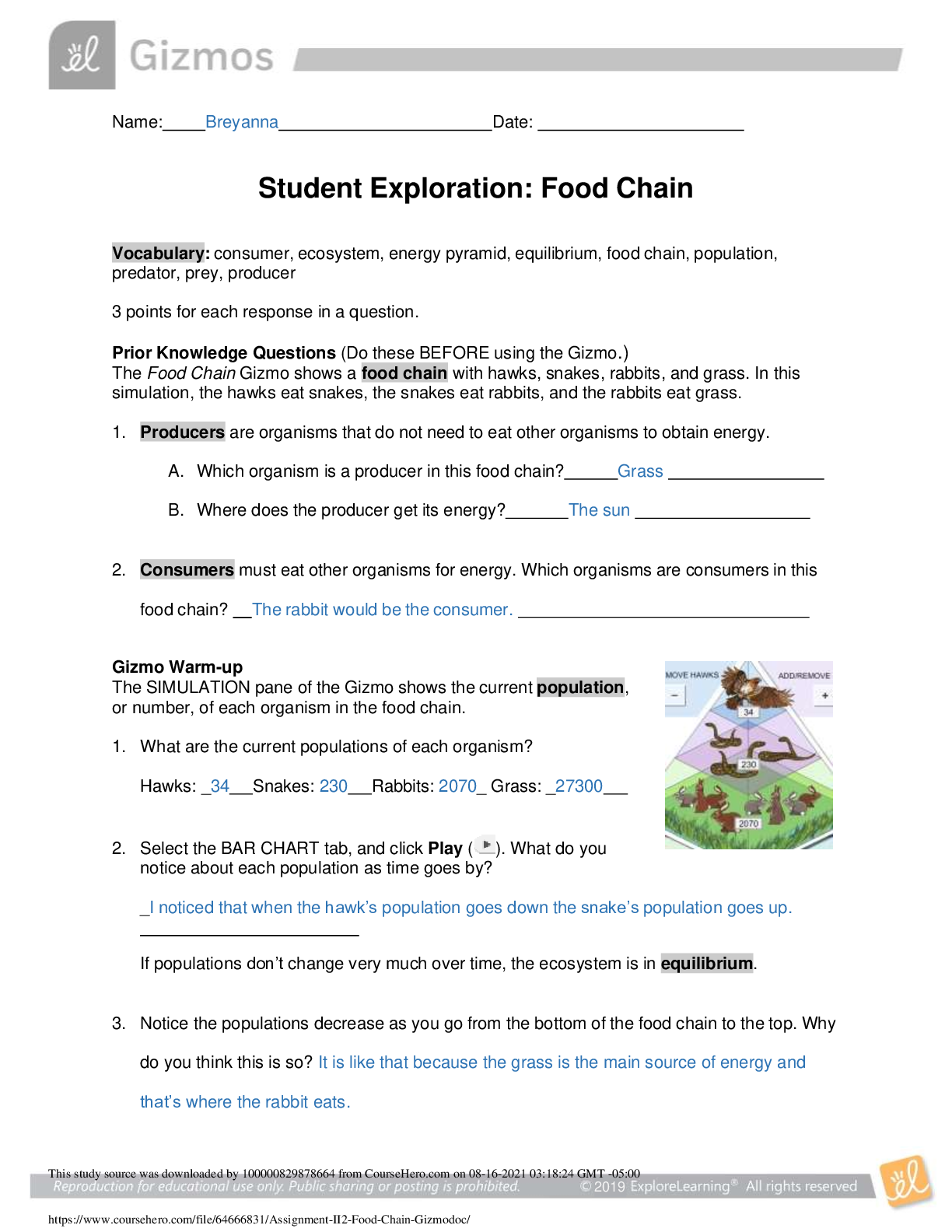
What do rice, potatoes, and sugar have in common?
They are all foods rich in carbohydrates. Carbohydrates
are an important energy source for your body. The basic
building block of most carbohydrate compounds is the
molecule glucose. Using the Dehydration Synthesis
Gizmo™, you will learn about the structure of a glucose
molecule and how glucose molecules can be joined
together to make larger carbohydrate molecules.
To begin, select the CREATE GLUCOSE tab.
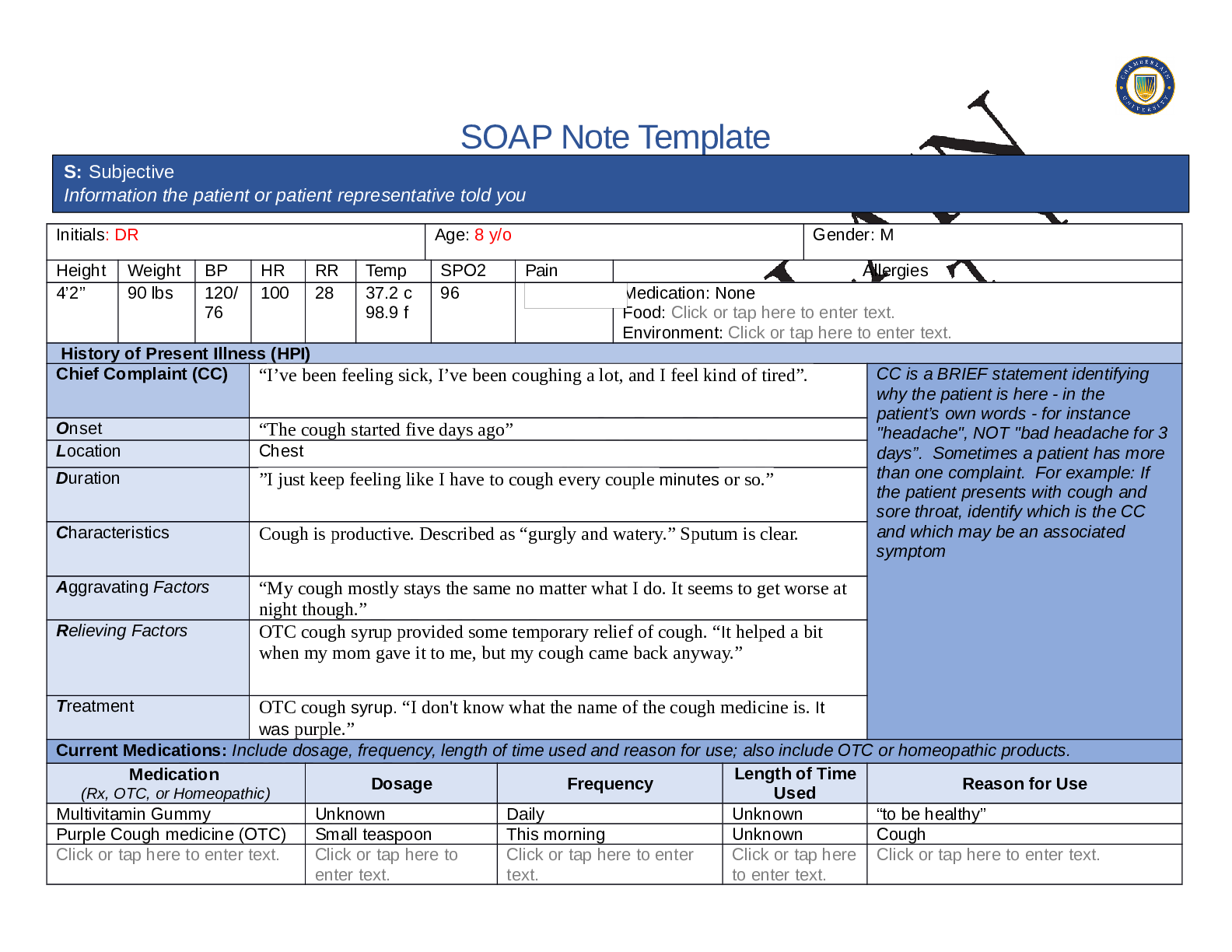
1. Look at the chemical formula for glucose. How many carbon (C), hydrogen (H), and
oxygen (O) atoms are found in a molecule of glucose? C:___6____ H:___12____
O:___6____
2. Turn on Show chemical structure. Each black sphere represents a carbon, hydrogen,
or oxygen atom. The lines connecting the spheres represent chemical bonds.
A. How many black spheres are in the diagram? 24
This study source was downloaded by 100000824015019 from CourseHero.com on 04-19-2021 18:17:16 GMT -05:00
https://www.coursehero.com/file/43923472/Assignment-II5-Dehydration-Synthesis-GIZMOdocx/
This study resource was
shared via CourseHero.comB. How does this relate to the number of carbon, hydrogen, and oxygen atoms in the
chemical formula for glucose? If there are 24 spheres, the equation when you split
them can be 6:12:6 just like it says it should be.
This study source was downloaded by 100000824015019 from CourseHero.com on 04-19-2021 18:17:16 GMT -05:00
https://www.coursehero.com/file/43923472/Assignment-II5-Dehydration-Synthesis-GIZMOdocx/
This study resource was
shared via CourseHero.comActivity A:
Build a glucose
molecule
Get the Gizmo ready:
Be sure the CREATE GLUCOSE tab is still
selected.
Introduction: Each element tends to form a certain number of chemical bonds. This value is
the valence of the element. For example, a carbon atom has a valence of four.
Goal: Construct a molecule of glucose.
1. Identify: The structure of a water molecule (H2O) can be written as H-O-H, with each dash
representing a chemical bond. Count the number of bonds the oxygen and hydrogen atoms
form in a water molecule.
A. What is the valence of oxygen? 1
B. What is the valence of hydrogen? 2
2. Build a model: Use the carbon, oxygen, and hydrogen atoms from the Atoms box to build a
glucose molecule on the empty hexagon in the building region. Use the chemical structure in
the lower right as a guide, and pay attention to the valence of each atom as you build.
Once you think you have correctly constructed the glucose molecule, click Check. If
necessary, continue to modify your molecule until it is correct.
3. Make a diagram: Congratulations, you have completed a molecule of glucose! Click the
COPY SCREEN button to take a snapshot of your completed molecule. Paste the image
into a blank document and label the image “Glucos
[Show More]