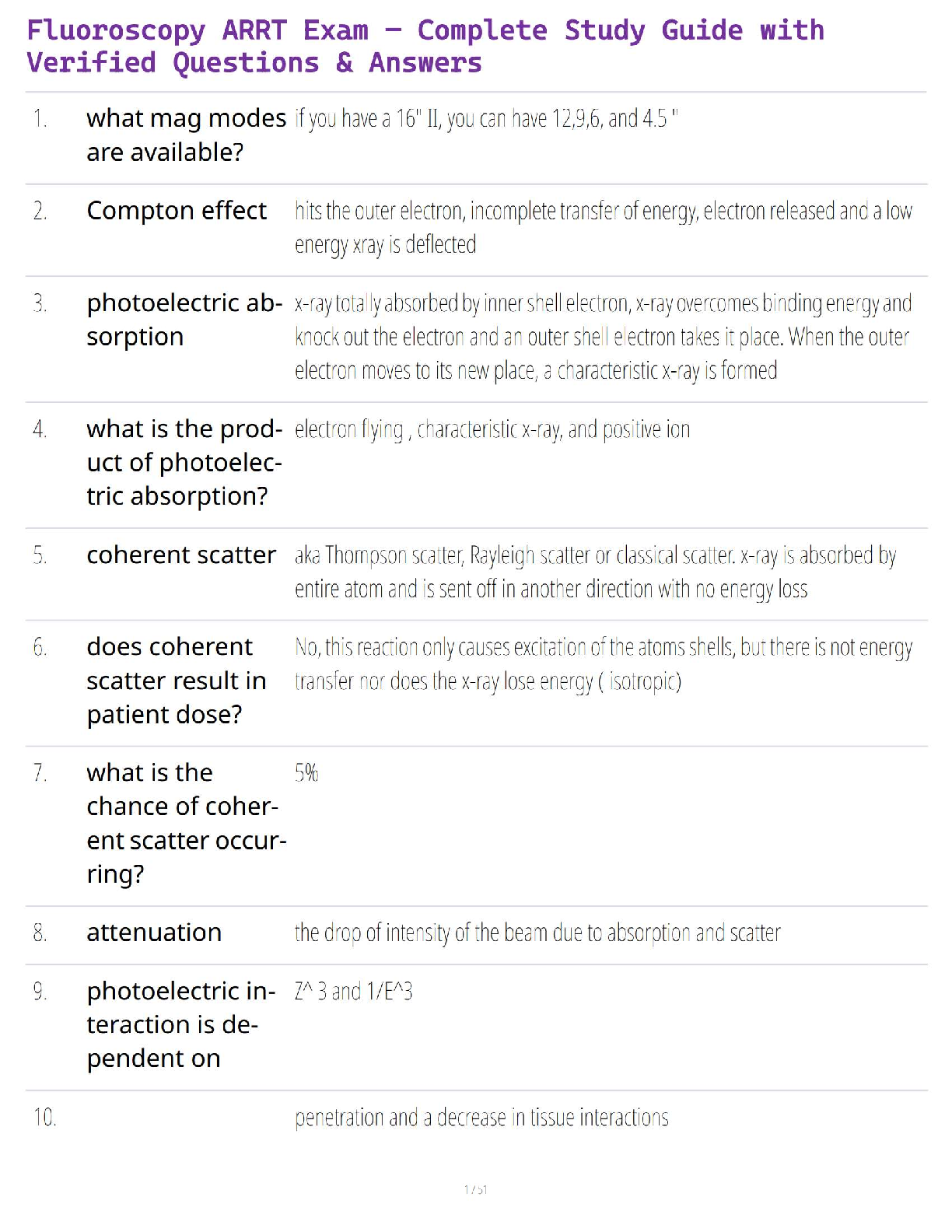
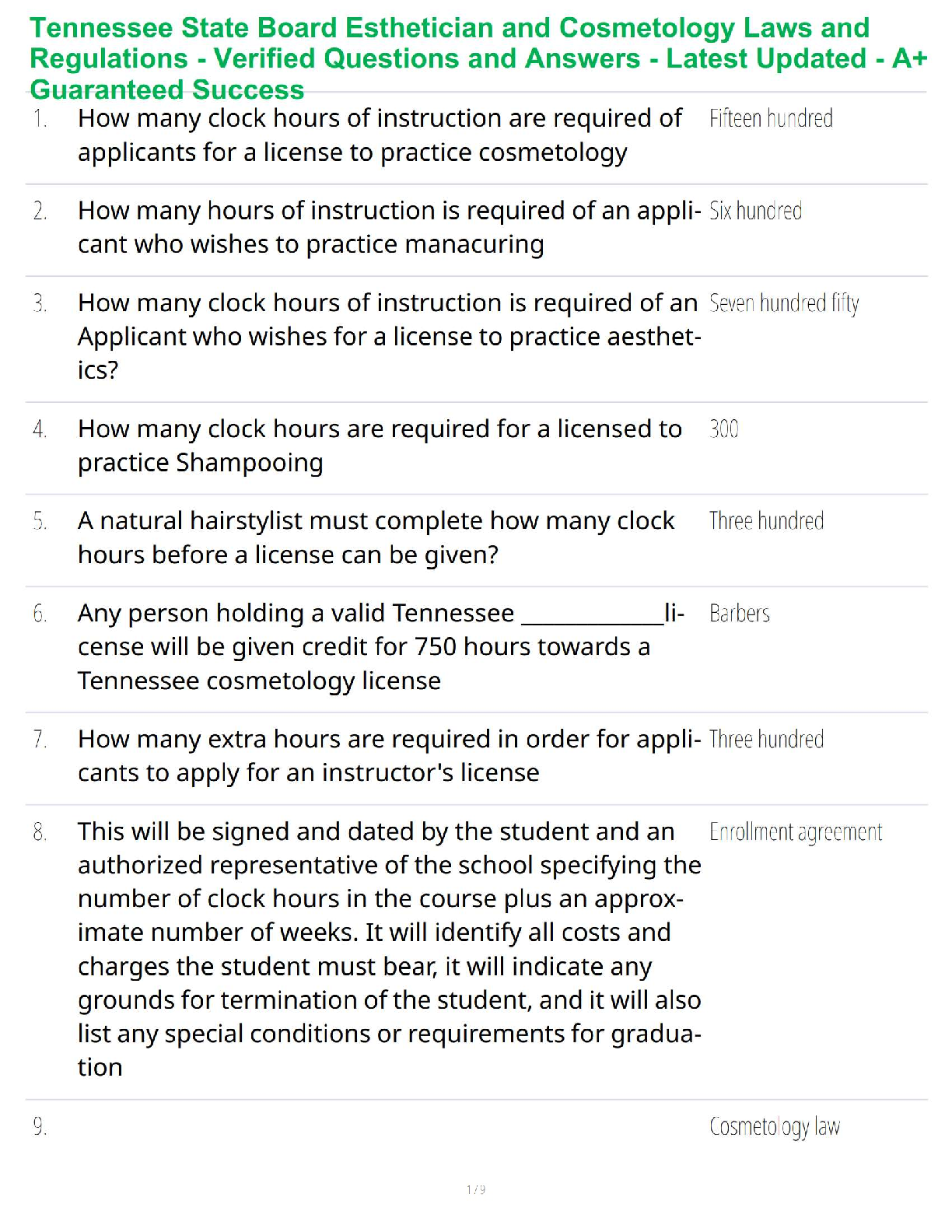
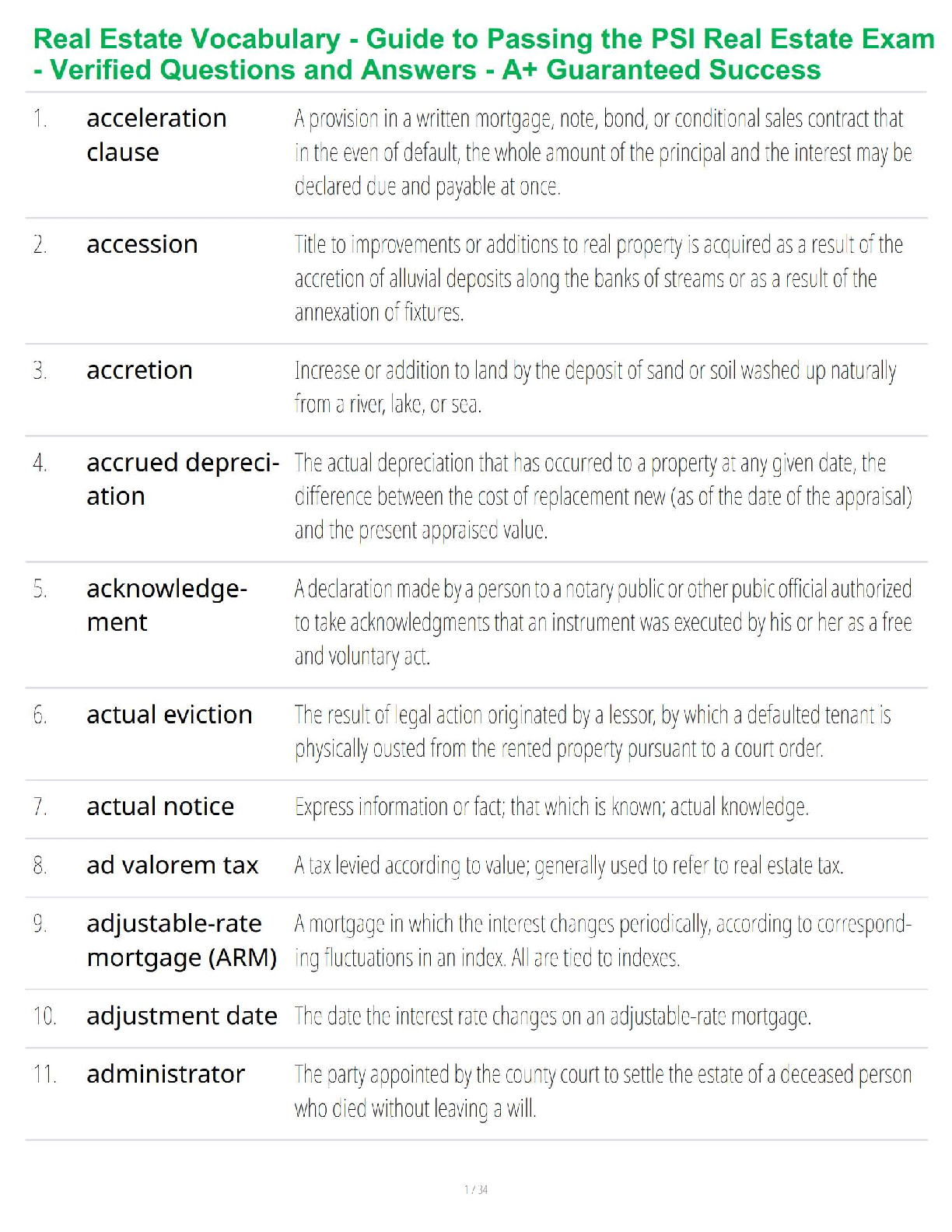
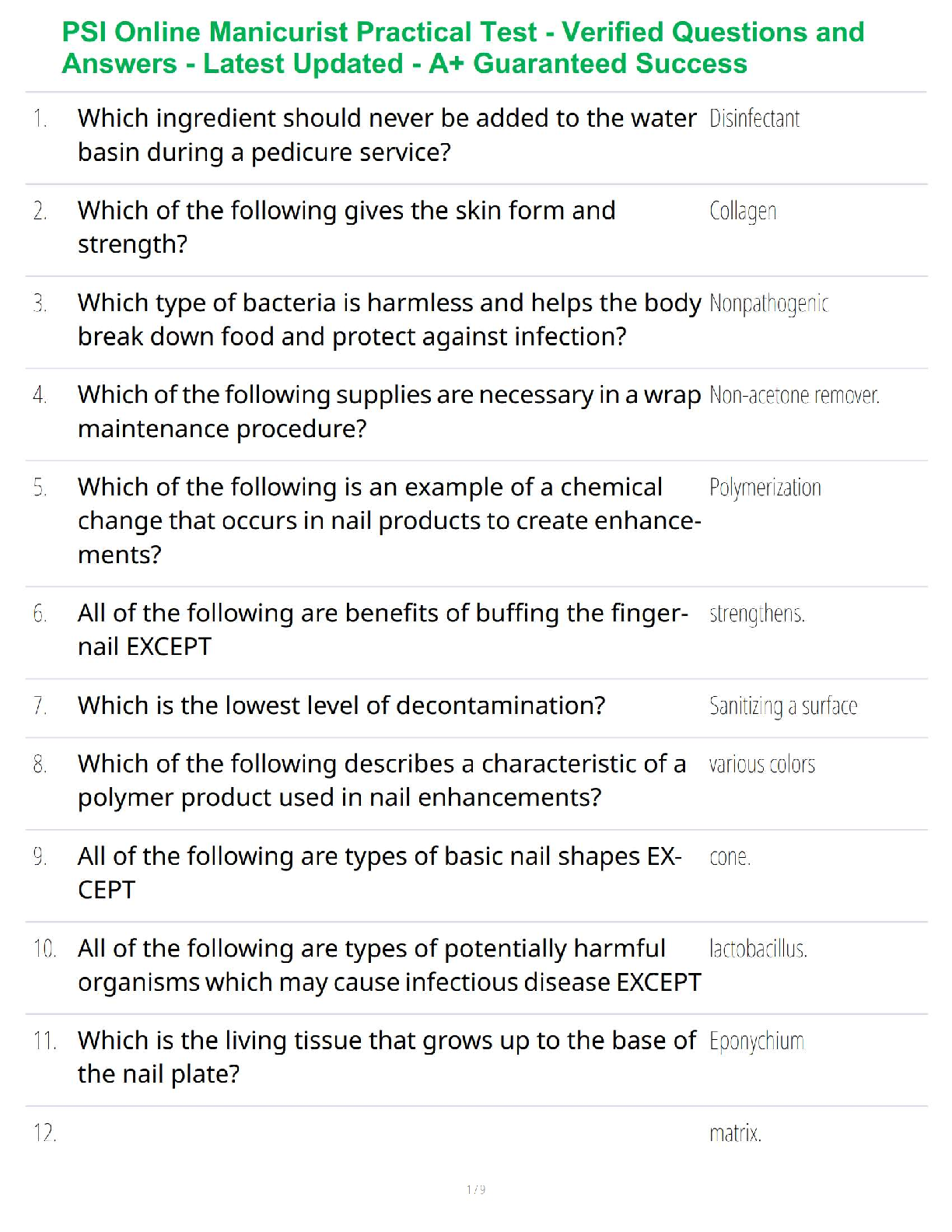
Balance the equation: C3H8 + O2 → CO2 + H2O (6 points)
Reactants:
C: 3
H: 8
O: 6
Products:
C: 3
H: 8
O: 6
C3H8 + 4 O2 → 3 CO2 + 4 H2O.
For the balanced chemical reaction: 2 HCl + Mg(OH)2 → MgCl2 + H2
...
Balance the equation: C3H8 + O2 → CO2 + H2O (6 points)
Reactants:
C: 3
H: 8
O: 6
Products:
C: 3
H: 8
O: 6
C3H8 + 4 O2 → 3 CO2 + 4 H2O.
For the balanced chemical reaction: 2 HCl + Mg(OH)2 → MgCl2 + H2
(a)Determine the MW of MgCl2. Show your work. (3 points)
(b) Determine how many mols of HCl you would require to produce 20 grams of MgCl2. Show your work (3
points)
(a) (Mg: 24.305g) + (Cl2: 35.45 * 2) = 95.211 g/mol
(b) 20 g MgCl2 * (1 mol MgCl2 / 95.211 g MgCl2) * (2 mol HCl /1 mol MgCl2) = 0.42 mol HCl
How many ml of a 8 M KBr solution are required to produce 250 ml of a 2 M KBr solution?
C1= 8 M
C2= 2 M
V2= 250 ml
V1:
250 ml * 2 M / 8 M
V1= 62.5 ml.
An isotope has a mass number of 67 and 39 neutrons. Determine the number of protons (2 points), number of
electrons (2 points) and the the chemical symbol of this isotope (2 points).
28 protons
28 electrons
Ni
Your daughter is an avid jump roper. The current brand that she uses, ACE, wears out within 1 week and has
recently worn out. You want to find a brand that lasts longer. Given the recent resurgence in this sport, there
are a number of manufacturers claiming to have high performance jump ropes. Teammate 1 says that brand
TTT "the best!”, Teammate 2 says that brand ZZZ "is cool!", and you also found a brand BBB jump rope at
the discount store. Use the scientific method to approach this scenario. Formulate a testable hypothesis (2
points). Design an experiment to test your hypothesis, and include your controls and be specific in your
experimental design (4 points). Predict the experimental results that would support your hypothesis (
[Show More]