CHEM 120 Unit 3 Quiz /CHEM 120 Unit 3 Quiz (100% Correct Solutions).
Document Content and Description Below
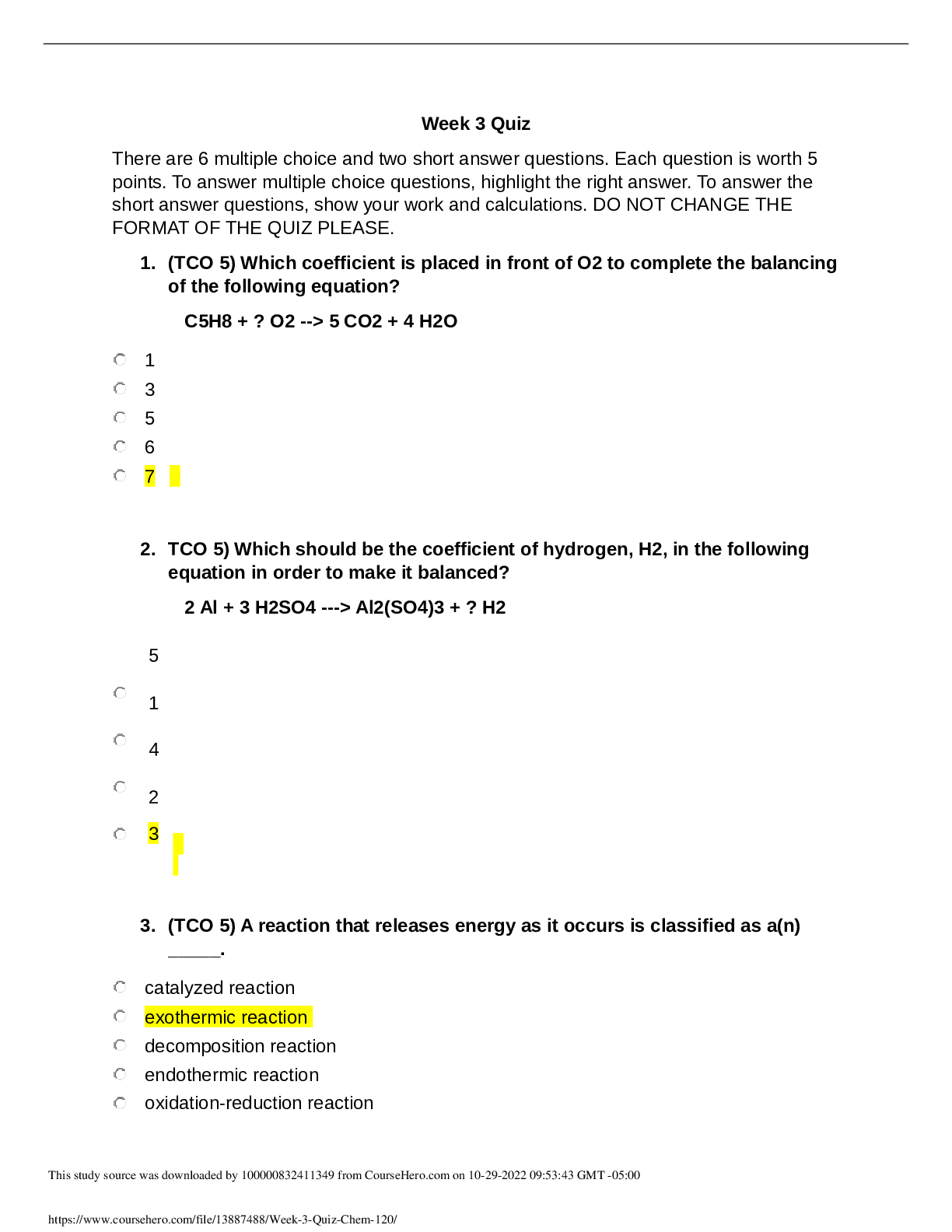
Week 3 Quiz There are 6 multiple choice and two short answer questions. Each question is worth 5 points. To answer multiple choice questions, highlight the right answer. To answer the short answer qu... estions, show your work and calculations. DO NOT CHANGE THE FORMAT OF THE QUIZ PLEASE. 1. (TCO 5) Which coefficient is placed in front of O2 to complete the balancing of the following equation? C5H8 + ? O2 --> 5 CO2 + 4 H2O 1 3 5 6 7 2. TCO 5) Which should be the coefficient of hydrogen, H2, in the following equation in order to make it balanced? 2 Al + 3 H2SO4 ---> Al2(SO4)3 + ? H2 5 1 4 2 3 3. (TCO 5) A reaction that releases energy as it occurs is classified as a(n) . catalyzed reaction exothermic reaction decomposition reaction endothermic reaction oxidation-reduction reaction 4. (TCO 5) Which of the following factors will increase the rate of a reaction? Adding more reactants Increasing the temperature of the reaction Adding a catalyst Neither A, B, nor C A, B, or C 5. (TCO 5) Any reaction that releases 150 kcal of energy can be classified as . activated exothermic endothermic oxidation reduction 6. (TCO 5) Magnesium reacts with oxygen to form magnesium oxide and has the following balanced chemical equation: 2 Mg + O2 --> 2 MgO. How many mole(s) of oxygen gas (O2) are needed to react with 4.0 moles of Mg? 2.0 moles 3.0 moles 6.0 moles 1.0 mole 4.0 moles 7. (TCO 7) What volume (L) of a 3M KOH solution can be prepared by diluting 0.5 L of a 5M KOH solution? Show your work. C1M1 = C2M2 0.5* 5 = 3* =V V = 2.5L/ 3 V = 0.83 L 8. (TCO 7) What volume (L) of a 2M KOH solution can be prepared by diluting 1.5 L of a 5M KOH solution? Show your work. C1M1 = C2M2 1.5*5 = 2*V V = 7.5/2 V = 3.75 L [Show More]
Last updated: 2 years ago
Preview 1 out of 3 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 30, 2021
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Nov 30, 2021
Downloads
0
Views
86




 Questions and Answers 100% VERIFIED.png)
 Questions and Answers 100% correct Solutions.png)






.png)