BioChemistry > STUDY GUIDE > BIOL 3362 - Exam 2 Study Guide (Biochemistry, DNA). (All)
BIOL 3362 - Exam 2 Study Guide (Biochemistry, DNA).
Document Content and Description Below
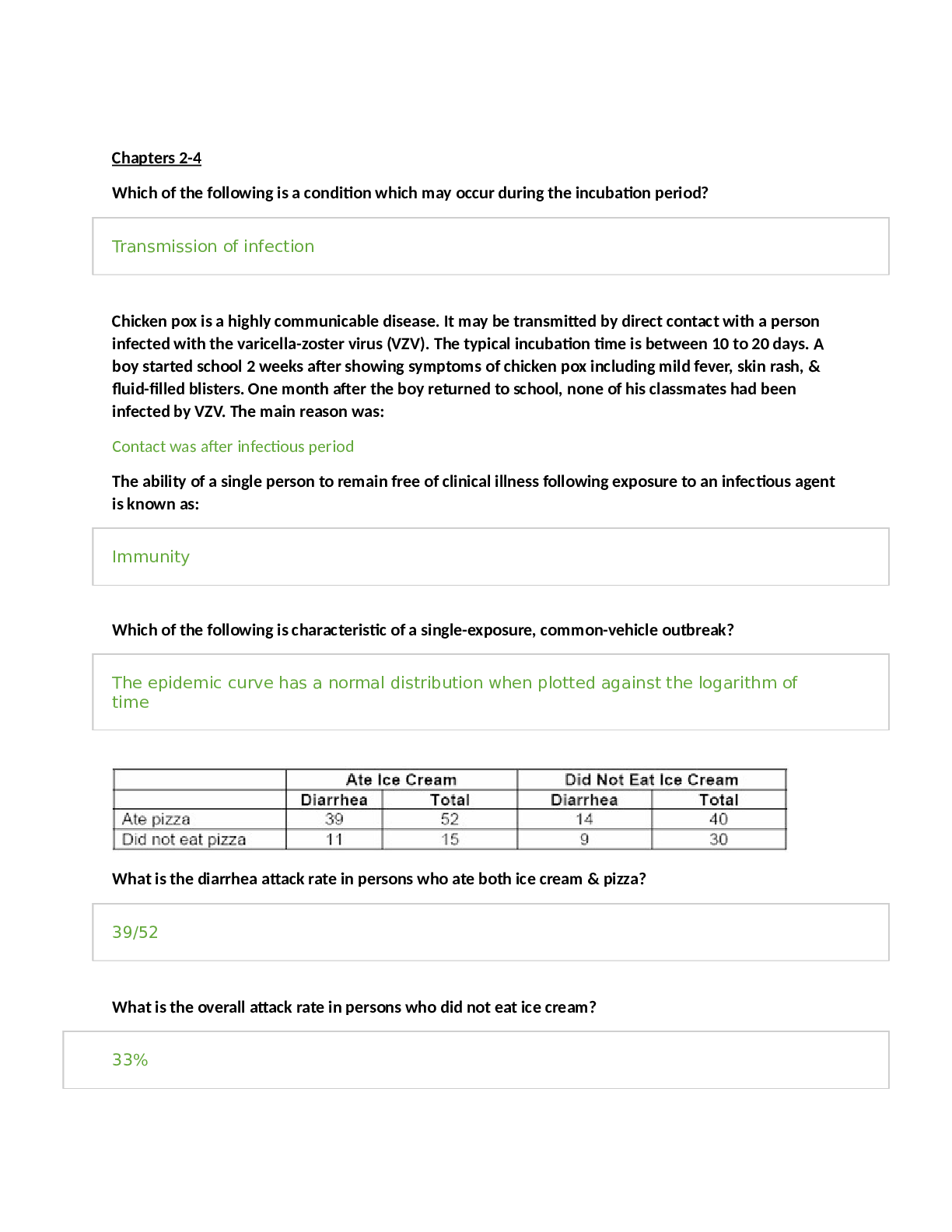
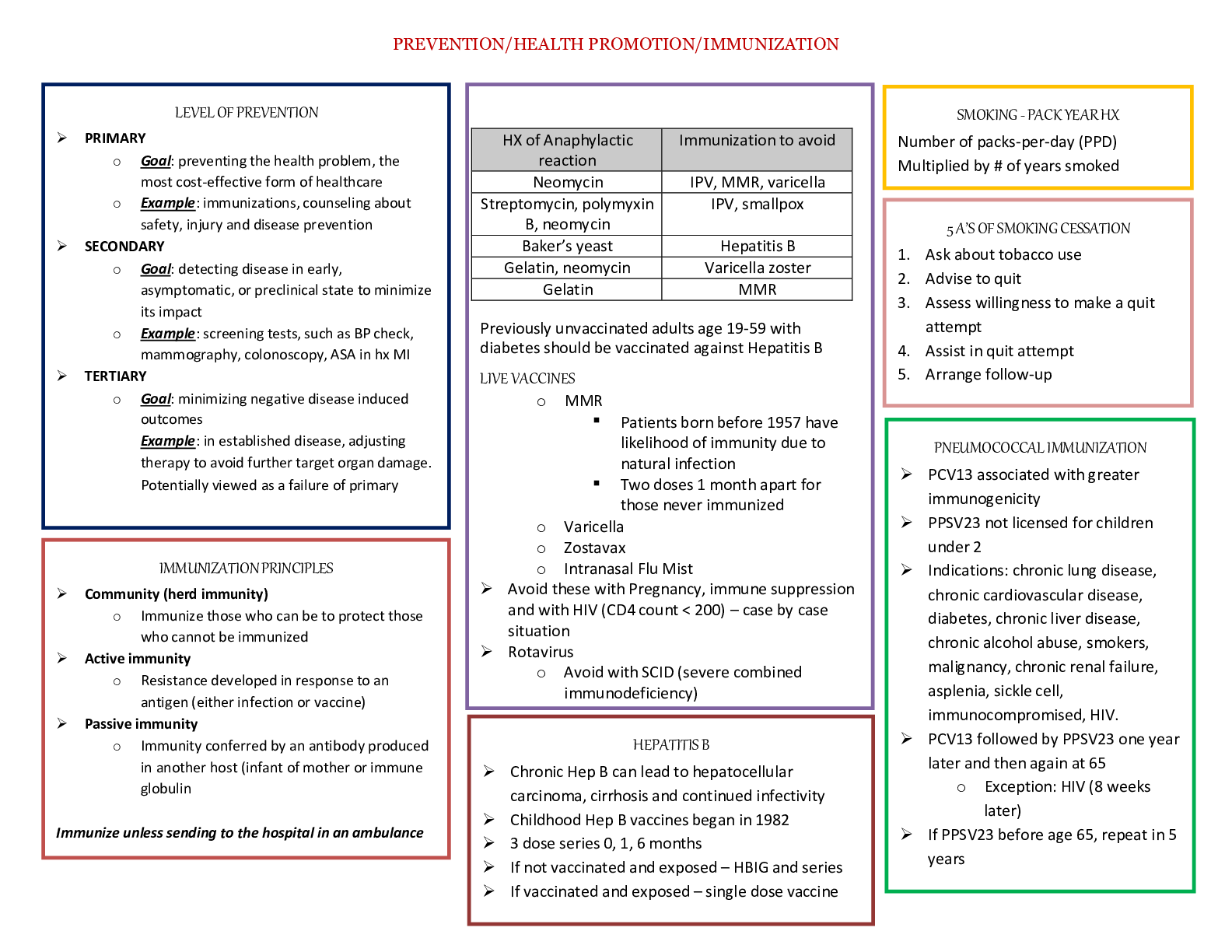
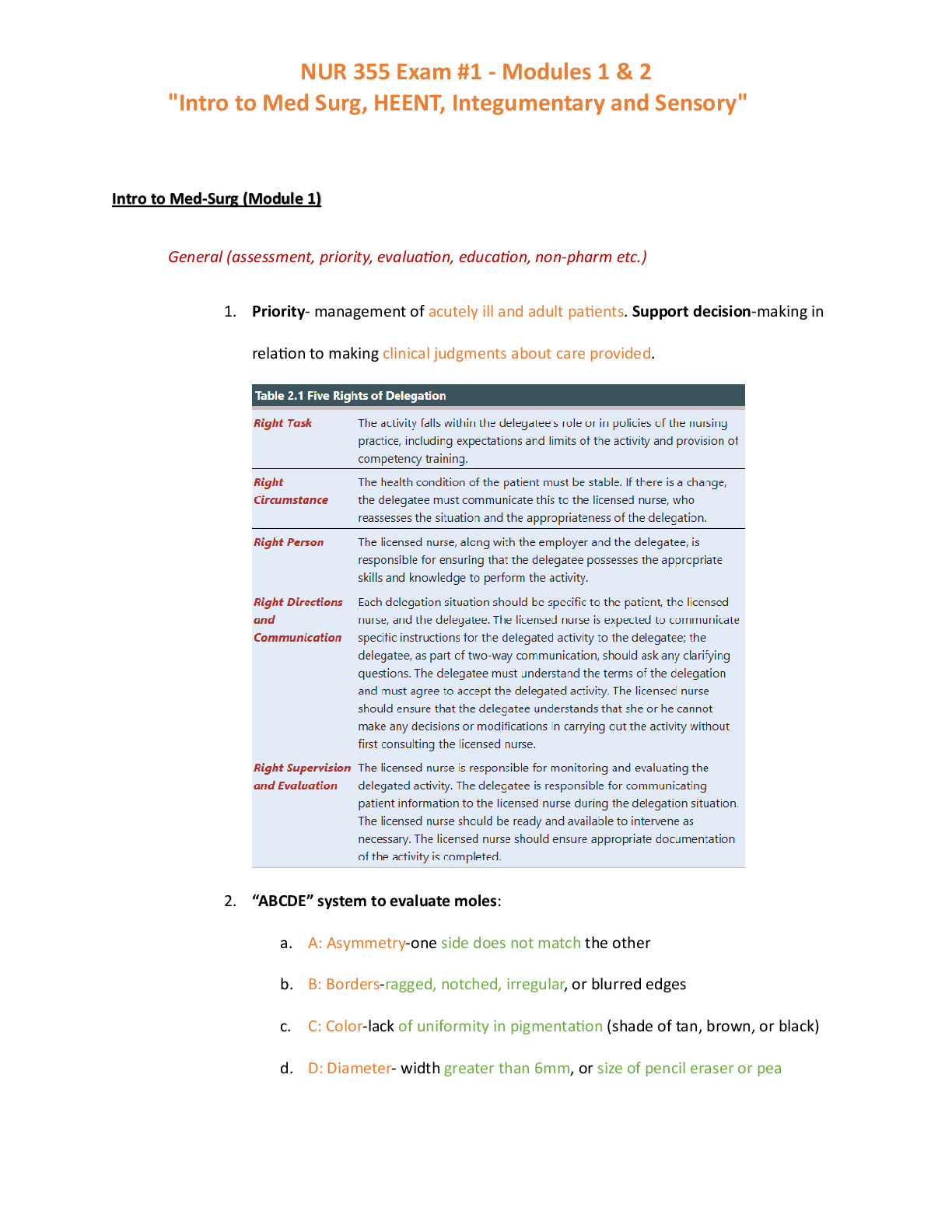
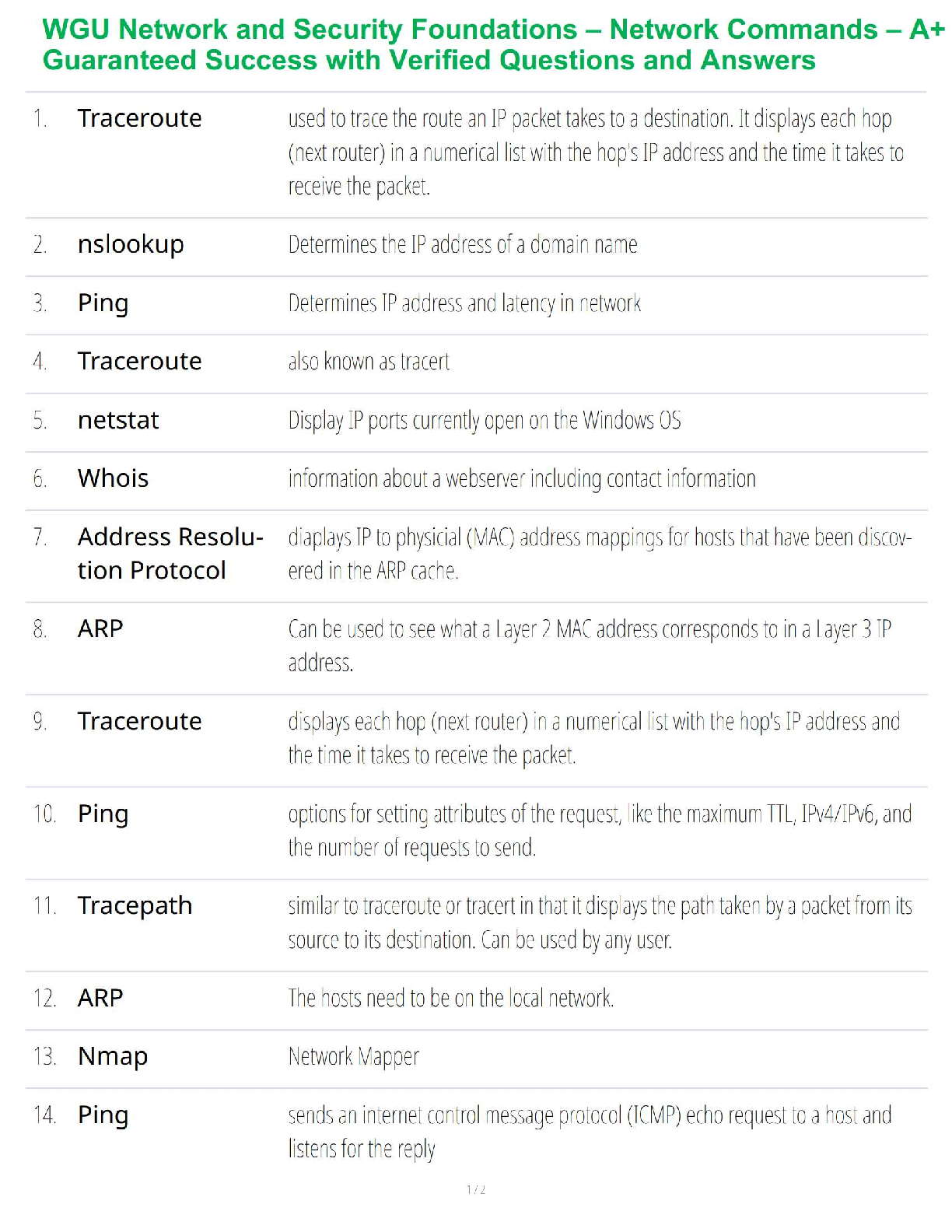
Study guide test 2 Strategies of nucleotide synthesis: precursors, when is ribose added, how is ribose activated and added, what are first purines and pyrimidines made, how are the various forms of ... THF made and/or interconverted, regulation of purine synthesis (where are committed steps), how is a balance between AMP and GMP achieved, how do folic acid antagonists impair purine synthesis. • Pyrimidines: base is made before adding the ribose sugar • Purines: ribose is already present sugar is activated bases are built on the sugar • • 2 General strategies of nucleotide synthesis : o 1. De novo pathway – from precursors ▪ Nucleotides are synthesized from “scratch” ▪ Bases are synthesized onto an activated ribose o 2. Salvage pathways – from nucleo-bases (bases w/ no ribose or phosphates) ▪ Energetically more favorable reaction ▪ An activated ribose is added to pre-existing nucleo-bases o (these pathways are usually targets for antibiotic and anticancer drugs) • De novo Pathway – Purine Precursors: o o Pathway of Purine synthesis – 3 major parts to make IMP (the first “purine”): ▪ 1. Activate ribose (step 1) • Ribose-5-phosphate pyrophosphokinase catalyzes pyrophosphate addition to the C-1 of ribose to form 5-phosphoribosyl-α- pyrophosphate (PRPP) (a 5- membered ring) ▪ 2. Synthesize 5-membered ring (steps 2-5) • i. N from Glutamine is added to PRPP (1st committed step) to form phosphoribosyl-amine o subject to feedback inhibition by AMP, ADP, ATP, GMP, GDP, and GTP (that is all adenine and guanine nucleotides) • ii. Precursors (Glycine, N-formyl-THF, Glutamine) are added to synthesize 5 membered ring o Addition of glycine requires ATP; glycine adds 2 carbons + 1 N o N-formyl-THF adds formyl group at the oxidation state of an aldehyde in preparation for cyclization of 5 membered ring o Nitrogen from Glutamine replaces an oxygen ▪ Antitumor drug azaserine is a glutamine analong that inhibits all glutamine-dependent enzymes by covalently attaching to nucleophilic groups in the glutamine-binding site. • antimetabolite • (inhibits step 5) ▪ 3. Synthesize 6-membered ring (steps 6-11) • ii. Precursors (Aspartate, N-formyl-THF) are added to synthesize 6 membered ring o ATP-dependent cyclization (ring closure) forms imidazole ring o ATP-dependent CO2 addition o ATP-dependent amide formation with aspartate as a N donor o N-formyl-THF adds formyl group at the oxidation state of an aldehyde in preparation for cyclixzation o Last step: cyclization is driven by dehydration ▪ 6 ATP consumed; 7 P-P bonds broken • Results in IMP (inosine monophosphate) – First purine made o Nucelotide because it has a phosphate + sugar o Pathway of Purine synthesis cont. – IMP conversion to AMP and GMP ▪ AMP synthesis • O on IMP is replaced with N from Aspartate • Uses GTP as energy ▪ GMP synthesis • O is added to IMP followed by an ATP-dependent replacement of the oxygen with a N from Glutamine • Uses ATP as energy ▪ Purine Balance: • When GMP is high, GTP will be high and drive AMP synthesis • When AMP is high, ATP will be high and drive GMP synthesis ▪ Regulation at the IMP branch: • AMP inhibits the first step in IMP to AMP • and GMP inhibits the first step in IMP to GMP o Regulation: ▪ PRPP also activates the second step by feed-forward activation • Activates the enzyme that consumes PRPP o Only activated when PRPP accumulates ▪ PRPP synthesis is regulated by ADP and GDP ▪ Phosphoribosyl-amine synthesis is regulated by AMP, ADP, ATP, GMP, GDP, and GTP ▪ AMP and GMP inhibit their own synthesis in [high] • Antagonists of Folic Acid Metabolism: o Affect N-formyl-THF additions of the purine synthesis pathway ▪ Folate THF • If folate synthesis is inhibited so is THF synthesis which is required for purine synthesis o Interconversions of THF in different oxidation states ▪ Interconverted by NADPH o Sulfonamides: ▪ Antimicrobial; hyperallergenic ▪ Folic acid antagonists because they look similar to PABA ▪ inhibits the conversion of PABA to folic acid ▪ Animals require folic acid, but bacteria have the pathway to make it, so they are more susceptible to sulfonamides that inhibit folic acid synthesis o PABA o Aminopterin: ▪ Rodenticide; teratogenic o Methotrexate: ▪ Anticancer agent; inhibits purine synthesis o Trimethoprim: ▪ Antibacterial, UTIs, pneumonia o 1st committed step 5 Formation of nucleoside di- and triphosphates, adenylylate (AMP) kinase and ATP generation in muscle. • Formation of diphosphates: o Use ATP as a phospho donor o Reversible reaction • Formation of triphosphates: o Nucleoside diphosphate kinase is a nonspecific enzyme that converts any nucleoside diphosphate (ribo or deoxyribo) to a nucleoside triphosphate with any nucleoside triphosphate as the phospho donor o Reversible reaction • The reaction AMP + ATP 2 ADP can run in the direction of ATP synthesis, and is used in muscle to generate energy. Purine salvage pathways: the role of PRPP, and the two major phosphoribosyltransferases, Lesch-Nyhan disease, gout and uric acid. • Purine Salvage pathways: o Salvage pathway takes the bases and adds an activated ribose-phosphate (PRPP) o Hypoxanthine = IMP without a sugar or a phosphate ▪ i.e. hypoxanthine + sugar + phosphate = IMP o Phosphoribosyl transferases catalyze the reaction to add a PRPP to a base ▪ 1. Adenine (APRT) • Used in adenine synthesis ▪ 2. Hypoxanthine-guanine (HGPRT) • Used in Hypoxanthine and guanine synthesis • complete deficiency of HGPRT results in Lesch-Nyhan syndrome o If HGPRT is blocked, PRPP accumilates and stimulates its consumption by feed-forward activation. This leads to an increase in nucleotide synthesis which elevated levels of the bases uric acid accumilation (gout); uric acid is insoluble o Lesh-Nyhan syndrome is a neurological diseases; produces mental retardation, aggressive behavior, and self-mutilation o o Nucleic acid degradation in intestine (fate of the base), and in cells; first common intermediate in degradation of all purines, the two reactions of xanthine oxidase, the use of allopurinol. • Nucleic acid degradation in intestinal tract o Nucleases and phosphodiesterases degrade ingested nucleic acids into nucleosides ▪ nucleosides are degraded by nucleosidases or nucleoside phosphorylases ▪ ribose can be used as carbohydrates for energy generation; the base is excreted (not absorbed) o The excreted bases are converted to Nucleotides (salvage pathways) o o The base is excreted, only the ribose is kept and used for purine synthesis • Nucleic acid degradation in cells o Nucleotidases convert nucleotides to nucleosides o The three purine nucleosides are converted by purine nucleoside phosphorylase to the bases hypoxanthine (from inosine), xanthine, and guanine. ▪ purine nucleoside phosphorylase adds a phosphate to ribose, which is released as ribose-1-P o Bases can be recycled when degraded then enter the salvage pathways o Bases generated largely by degradation inside cells • First common intermediate in purine degration: xanthine o Xanthine oxidase converts hypoxanthine xanthine o Guanine deaminase converts guanine xanthine • 2 Xantine oxidase reactions: o Converts hypoxanthine xanthine o Converts xanthine uric acid ▪ This reaction produces hydrogen peroxide (reactive oxygen species) ▪ This reaction is the greatest source of reactive oxygen species ▪ Humans and primates excrete uric acid, as a purine waste product • Birds, terrestrial reptiles and many insects excrete uric acid as the major mechanism to remove excess nitrogen. • Allopurinol – inhibits xanthine oxidase to prevent uric acid accumulation o Gout and uric acid accumulation: ▪ Gout – results from excess uric acid; one result is arthritis b/c of uric acid in joints (big toe is most susceptible) ▪ Uric acid can also generate kidney stones ▪ • SCID, adenosine deaminase deficiency, and resulting metabolic problems. • Adenosine deaminase deficiency and SCID o Difficency in the deamination of adenosine to inosine ▪ Results in SCID o Primary degradation pathway for adenine nucleotides o Degrades deoxyadenosine and adenosine (somewhat nonspecific) ▪ Enzyme only recognizes base, not the sugar so it cannot specificy between the two o Deoxyadenosine does not convert to deoxyinosine so deoxyadenosine accumilates ▪ Nucleoside kinase converts deoxyadenosine dAMP dATP • dATP inhibits conversion of ribonucleotides to deoxyribonucleotides and stalls DNA replication ▪ SCID affects the immune system; failure to have immune response o The function of the purine nucleoside cycle (anaplerotic reaction); “Futile cycle” • Energy generating pathway in muscle o Muscles use this mechanism to generate a citric acid cycle intermediate, since it lacks the normal anaplerotic enzymes that convert three-carbon intermediates to four-carbon intermediates • AMP can be deaminated to IMP o The IMP can be reconverted to AMP by the last two enzymes of AMP synthesis • net result of this cycle is a GTP-dependent conversion of aspartate to fumarate and NH4+. • Pyrimidine synthesis: precursors, first pyrimidine made, first cytosine derivative made, regulation, metabolic channeling • Pyrimidine = 6 membered ring with 2 nitrogens o o (purines have a pyrimidine ring in them + a 5 membered ring) • Precursors: Aspartate and Carbamoyl-Phosphate • Pyrimidines: pyrimidine ring is made before adding the ribose sugar o Purines: sugar is already present sugar is activated rings are built on the sugar • Carbamoyl-P: o Carbamoyl-Phosphate Synthetase II (CPS-II) synthesizes Carbamoyl-P in mammals ▪ CPS-II is in the cytoplasm; dedicated to pyrimidine synthesis o CPS-II uses glutamine as the nitrogen donor ▪ (Recall: CPS-I (in the mitochondria; part of urea cycle) uses NH3 as N donor) o CPS-II making Carbamoyl-P is the 1st committed step in pyrimidine synthesis in mammals ▪ In contrast, there is only one CPS in E. coli and other bacteria. Therefore the next step is the first committed step • Aspartate transcarbamoylase (ATCase) condenses carbamoyl-P with aspartate to form carbamoyl-aspartate. • o Dihydroorotate is donating electrons to coenzyme Q in the mitochondrial membrane to contribute to the e- transport chain ▪ (oxidation reaction) ▪ Couples pyrimidine synthesis to energy generation o A complex dehydrogenation produces a reduced intermediate, which in eukaryotes is a quinone. ▪ The product is orotate, which is a pyrimidine. o Ribose is added, with PRPP as the ribose donor, to form orotidine-5’ monophosphate o The last step is decarboxylation to form UMP (1st pyrimidine made) • Metabolic channeling (mammals only): o One metabolite is being handed to the next enzyme which hands it to the next, etc. o The first three enzymes (steps 1-3) in mammals are part of one polypeptide, with three active sites o The fourth enzyme (step 4) is associated with the outer surface of the inner mitochondrial membrane ▪ Note that the electron donor for the dehydrogenation is ubiquinone o The last two reactions are also catalyzed by a proteins with two active sites o multifunctional proteins are more efficient since they prevent diffusion of the metabolites away from the complex, which speeds up the reaction • UTP and CTP synthesis: o UMP UDP UTP ▪ UMP is phosphorylated to UDP by nucleoside monophosphate kinase ▪ UDP is phosphorylated to UTP by nucleoside diphosphate kinase o CTP is made from UTP by CTP synthetase with glutamine as the nitrogen donor ▪ Note the conversion at the NTP level o o (Recall): ▪ First Adenine nucleotide = AMP ▪ First Guanine nucleotide = GMP ▪ First Uridine nucleotide = UMP ▪ First Cytosine nucleotide = CTP** • Precursor: UTP o O is replaced by N from glutamine • Regulation: o In bacteria (E. coli): ▪ ATCase is feedback-inhibited by CTP • (in other bacteria, UTP feedbacks inhibits) ▪ ATP ( a signal of energy and purines) stimulates ATCase activity • Therefore, pyrimidines are not made until there are sufficient purines. Purines first. o In animals: ▪ first committed step is CPS-II (synthesis of carbomyl-P) and it is inhibited by UDP and UTP ▪ ATP and PRPP are allosteric activators o Deoxyribonucleotide synthesis and ribonucleotide reductase: source of reducing power, thioredoxin, substrates, binding of substrates to catalytic site, the two regulatory sites. • E. coli ribonucleotide reductase o 2’-OH on the ribose is converted to a 2’-H by ribonucleotide reductase ▪ The nucleotides are at the diphosphate level ▪ This enzyme replaces the 2’ hydroxyl with a hydride o • The overall process requires three proteins: ribonucleotide reductase, thioredoxin, and thioredoxin reductase • Electrons from NADPH are being transferred to reduce the 2’-OH 2’-H o NADPH provides the reducing power, which reduces components of thioredoxin reductase, which reduces thioredoxin, and finally the -SH groups of ribonucleotide reductase (thioredoxin reductase thioredoxin ribonucleotide reductase) ▪ Thioredoxin is a small protein with reactive cysteine sulfhydryl groups ▪ Thioredoxin is involved in several reversible sulfide:sulfhydryl transitions ▪ (electron transfer occurs through sulfhydryl groups that transfer electrons to the ribose) o Electron transfer for ribonucleotide reductase: ▪ • NADPH can also reduce glutathione which can reduce glutathione reductase and glutaredoxin (similar to thioredoxin) (glutathione glutathione reductase glutaredoxin) o Glutaredoxin can function in the ribonucleotide reductase reaction • Ribonucleotide reductase is an α2β2 enzyme: o ▪ subunit carries two types of regulatory sites and the catalytic site • catalytic site binds the substrates: ADP, GDP, CDP, and UDP o CDP and UDP are unusual ▪ CDP is not made during pyrimidine synthesis. Instead, it must be made from CTP ▪ UDP results in dUDP formation, and it is the precursor for dTTP • One regulatory site is the substrate specificity site o binds ATP, dATP, dGTP, and dTTP o determines which substrate binds to the catalytic site • Second regulatory site binds either ATP or dATP o ATP binding activates activity o dATP inhibits total activity ▪ The most predominant type of enzyme requires iron, and generates a free radical on a tyrosyl residue o Ribonucleotide reductase regulation part II (Regulation of deoxynucleotide biosynthesis) ▪ Regulated by the binding affinity at the two nucleotide-binding regulatory sites on ribonucleotide reductase ▪ Synthesis of dTMP: precursor, the THF derivatives (substrate and products), and the unusual reaction of a THF derivative, why is this enzyme a target of inhibitors of DNA synthesis and how do folic acid analogs block synthesis. • Precursor: dUMP dTMP • dTMP production is dependent on dUMP formation from dCDP and dUDP o Synthesis of dUMP for dTTP synthesis: o From dUDP: ▪ dUDP (the product of ribonucleotide reductase) is first converted to dUTP, which is then cleaved to dUMP by dUTPase • This strange route utilizes an enzyme that prevents dUTP incorporation into DNA o From dCDP: ▪ dCDP is dephosphorylated to dCMP, and then deaminated by dCMP deaminase which results in dUMP synthesis • dTTP inhibits dUMP synthesis from dCMP: standard endproduct inhibition • Next step is conversion of dUMP to dTMP by thymidylate synthase o reaction involves a reductive methylation, with N5,N10-methylene-THF ▪ The THF cofactor is itself oxidized, to yield dihydrofolate (DHF) o A separate enzyme reduces DHF THF, which can then accept another C1 unit in the conversion of serine glycine • Conversion of dUMP dTMP is a frequent target for inhibitors of DNA synthesis o Folic acid precursors or analogs will block this step (Ex. Sulfonamides) o Thymine analogs can also inhibit this reaction ▪ some metabolism may be required before inhibition** ▪ Ex: 5-fluorouracil (Anticancer agent sold as Adrucil) • 5-fluorouracil is converted to a nucleotide by a salvage enzyme, with PRPP as the phosphoribosyl donor • This is converted to its deoxy version by ribonucleotide reductase, and the product inhibits thymidylate synthase • Fluorine cannot be abstracted, and the enzyme does not turnover because the intermediate is poorly released. • o o Tautomerizations: how do they affect base pairing, how do they cause mutations • The common bases can undergo a shift in structure • A keto-enol (carbonyl-hydroxyl) tautomerization occurs for guanine and thymine o keto form predominates at neutral pH o Keto-enol tautomerization of Uracil: ▪ o Keto-enol tautomerization of Guanine: ▪ • An amino-imino tautomerization occurs for adenine and cytosine o amino form predominates at physiological pH • The shift in structure determines whether the nitrogen or oxygen is a hydrogen-bond acceptor or donor o The oxygens and nitrogens determine base-pairing properties • The rare form (enol and imino tautomers) base-pairs incorrectly, forming A:C and G:T base pairs o The tautomeric-based structural changes (enol and imino tautomers) are frequent causes of mutations. • Tautomerization structural properties can be used to induce mutations o Ex: Bromouracil - a base analog that can be incorporated into DNA ▪ tautomerizes easily - BrdU, which should normally base pair with A, frequently base pairs with G o Ex: Inosine also readily interconverts between tautomeric forms ▪ used in tRNA anticodons in the third position; accounts for why one tRNA can read more than one codon What bonds in nucleotides and nucleic acids are unstable and under what conditions? • RNA contains D-ribose, and DNA contains 2-deoxy-D-ribose o The deoxyribose (which has one less functional group) increases the stability of DNA relative to RNA because of differences in susceptibility to chemical and enzymatic hydrolysis o Know the difference between bases, nucleosides, nucleotides, and their names. • Bases: no sugar, no phosphate; only the ring • Nucleosides: base + sugar (linked via a glycosidic linkage) • Nucleotides: base + sugar + phosphate • The names of the nucleosides are derived by: o adding an “-osine” to the purine bases (adenosine, guanosine, inosine) o adding an “-idine” to the pyrimidines (cytidine, uridine, thymidine) o only exception is inosine its base is hypoxanthine • Nucleotides: nucleoside phosphates o nucleotide has a phosphate esterified to an hydroxyl group of the ribose ▪ For the common nucleotides, the esterification is at the 5’ OH o nucleotides are acidic (as suggested by their names: 5’-AMP is also called adenylic acid) o At neutral pH, the phosphate has two negative charges o Ribonucleotides: ▪ o Cyclic nucleotides: ▪ the phosphate is esterified to hydroxyls, such as cyclic AMP, which is more correctly called 3’,5’-cyclic AMP ▪ cyclic nucleotides usually have just a regulatory function ▪ ▪ o Nucleoside diphosphates and triphosphates: ▪ Additional phosphates can be added via formation of phosphoric anhydride linkages • additional phosphates are designated by the Greek letters , , and , with the being the closest to the ribose o The and phosphates are easily hydrolyzed by acid o Additional phosphates are added by a dehydration synthesis reaction ▪ Ex: Formation of ADP and ATP ▪ Phosphoric anhydride bonds are the primary carriers of chemical energy; Each of the nucleotides has a distinct function: • ATP for energy transactions • GTP for protein synthesis • CTP for phospholipid synthesis • UTP for oligosaccharide synthesis • (each of these are also precursors for nucleic acids) • The phosphates contribute to the energy transactions; The bases do not participate in the covalent bond chemistry, but apparently have a function in recognition of the appropriate enzymes ▪ Reactions of nucleotides: ▪ NDPs and NTPs are polyprotic acids: • NDPs dissociate into 3 protons • NTPs dissociate into 4 protons • The resulting anions can form stable complexes with Mg2+ or Ca2+ o Mg2+ concentrations are high so the NDPs and NTPs form complexes with Mg2+. The glycosidic linkage and its stability. • Nucleosides are bases linked to a sugar via a glycosidic linkage • A glycosidic bond is a linkage via the carbonyl carbon of the linear structure which is linked to the oxygen atom that is part of the cyclic structure • The carbon atom involved in the glycosidic linkage is called the anomeric carbon o The linkage is an N-glycosidic linkage because the anomeric carbon is attached to the N-1 of the pyrimidine or the N-9 of the purine o The linkage is in the -configuration (above the ring) for all nucleosides and nucleotides • Nucleosides are more soluble than the bases because of the ribose o In base: The glycosidic linkage in nucleosides is stable in alkali o In acid: the purine glycosidic linkage is not stable, but the pyrimidine linkage is stable Anti and syn configurations: what structure is not allowed and why? • The base can rotate around the C-N glycosidic linkage, but this is hindered by the hydrogen of the ribose C-2’ carbon o permits two conformations: anti and syn ▪ Anti: purine ring is below the ribose; base below the sugar ▪ Syn: purine ring is above the ribose; base over the sugar o Purine nucleosides and nucleotides usually adopt the anti conformation • Pyrimidines are always anti, never syn, because the 2-O atom of pyrimidines sterically hinders the ring from a position above the ribose o Pyrimidines favor the anti configuration because the oxygen of the pyrimidine gets in the way • Purines can adopt either the syn or anti configuration The biological function of adenosine, effects of caffeine: • Adenosine acts as a local hormone or autocoid • Adenosine circulates in the bloodstream and influences: blood vessel dilation, smooth muscle contraction, neuronal discharge, neurotransmitter release, and fat metabolism o Hard-working muscle cells release adenosine, which dilates blood vessels and increases blood flow, O2 delivery, and nutrient delivery o Adenosine also regulates heartbeats, by blocking the electrical current that controls the heart, which slows heart beats • Elevated levels of adenosine induces sleepiness o Caffeine counteracts this effect by blocking the interaction of adenosine with the neuronal receptors Conventions for writing polynucleotides sequences and structures. • Nucleic Acids are Polynucleotides: o Linear polymers are formed by successive addition of 5’-nucleoside monophosphates to the 3’-OH of the preceding nucleotide o A pentose is the simplest possible structure for polynucleotide formation ▪ C-1’ and C-4’ are involved in furanose formation leaving only the C-3’ and C-5’ for polymer formation Classes of nucleic acids and their properties. What are unique structures or components of each type of RNA? • DNA: o Double helix with anti-parallel strands; base pairs held together with hydrogen bonds o Chargaff noticed certain rules in DNA: ▪ Noticed that A:T and G:C were always in a 1:1 ratio suggesting that they base-paired with eachother • Also noticed a 1:1 purine to pyrimidine ratio suggesting that a balance is required o Watson-Crick proposed base-pairing; lengths of the A:T and G:C pairs are virtually identical ▪ A:T = 2 H-bonds (1.11 nm) ▪ G:C = 3 H-bonds (1.08 nm) o Replication (and transcription) are based on base pairing o E. coli: ▪ chromosome is 2 nm in diameter, but 1,600,000 nm in length; highly folded in cells ▪ bacterial chromosome is circular, and there is only one in E. coli (some bacteria have more than 1) o Eukaryotic chromosomes are condensed with proteins, such as the arginine- and lysine-rich histones • RNA: o Three basic types of RNA (rRNA is most abundant) ▪ rRNA, tRNA, mRNA (in order of most least abundant) o Other forms of RNA: snRNA in eukaryotes, tmRNA in prokaryotes o mRNA: ▪ Made from one strand ▪ In prokaryotes, a single mRNA can code for several proteins (not eukaryotes) • PolyA tail promotes degradation ▪ Eukaryotic mRNA is made initially as hnRNA which is processed to smaller forms (after splicing) • introns are removed, and exons remain to be translated • Eukaryotic mRNAs have a run of 100-200 As (polyA tail) that add stability o PolyA tail added after transcription o rRNA: about 65% of the weight of ribosomes ▪ rRNA is highly folded because of intramolecular hydrogen bonds ▪ ▪ size of ribosomes (and large complexes in general) is most easily measured by sedimentation (which is actually a measure of size and shape) ▪ Eukaryotic and prokaryotic ribosomes differ in size. Each consists of two subunits, which are held together by Mg++ ▪ Bacteria: • large 50S bacterial subunit contains a 5S and 23S rRNA • small 30S subunit has a 16S rRNA ▪ Eukaryotes: • three rRNAs in the large subunit ▪ Ribosomes contain a number of unusual bases o tRNA: ▪ carriers of amino acids for protein synthesis ▪ highly folded due to intramolecular hydrogen bonding ▪ contain from 73 to 94 residues; Many of the bases are modified ▪ amino acid is attached at the 3’ end • sequence of the end is CCA • amino acid is added to the 3-OH of the terminal adenine residue ▪ Some amino acids may have up to five different tRNAs ▪ There are different tRNAs in each compartment (mitochondira versus cytoplasm) o snRNAs: small nuclear RNAs ▪ contain 100-200 nucleotides (many are modified) ▪ abundant in the nucleus of eukaryotes (1-10% of number of ribosomes) ▪ associated with proteins, and participate in splicing o small RNAs: ▪ 21-28 nucleotides ▪ can bind to DNA or RNA by complementarity ▪ RNA interference (RNAi) is mediated by small interfering RNAs (siRNA) • double-stranded regions are targeted for degradation • Can be used for gene silencing; RNAi also contributes to chromatin structure ▪ The micro RNAs (miRNAs) control developmental timing • do not control RNA degradation, even though they bind targets • Differences between DNA and RNA: o Two differences: Deoxyribose vs Ribose, and Thymine vs Uracil ▪ These differences make DNA more stable than RNA for two reasons • 1. cytosine deaminates to uracil: o This change would alter base pairing properties, and results in a mutation o U is efficiently removed from DNA o Thymine is methylated uracil, and is not removed ▪ T and C are not interconvertible • 2. the deoxyribose (loss of the 2’-OH) results in greater resistance to alkaline hydrolysis o the 3’-phosphodiester bond is normally susceptible to cleavage Nucleic acid stability in acid and alkali. Nucleic acid hydrolysis and nuclease specificity: where does cleavage occur? • Nucleic Acid Hydrolysis: Hydrolysis often breaks the phosphodiester backbone • Acid hydrolysis: o 1 mM HCl hydrolyzes the purine glycosidic linkage in DNA, but not in RNA o phosphodiester bond is intact, but a gap exists in the DNA • Alkaline hydrolysis: o DNA is stable (as discussed above); RNA is not o The phosphate may end up on the 2’ or the 3’ carbon of ribose. • Enzyme hydrolysis: o Nucleases degrade nucleic acids ▪ These enzymes are found in all cells ▪ Snake venom contains a potent phosphodiesterase, which breaks the phosphodiester backbone ▪ Cleavage can occur on either side of the phosphate • convention labels the 3’ side as a, and the 5’ side as b ▪ Nucleases are also catagorized as endo (internal) or exo nucleases • An exo nuclease removes the bases at the ends • Nuclease specificity: o Some nucleases attack only DNA or RNA (some can attack both) o Nucleases can be specific toward single- or double-stranded nucleic acids o Other nucleases require binding to a specific sequence, such as restriction enzymes o Restriction enzymes are specific endonucleases ▪ The term “restriction” comes from the property that it protects bacteria from foreign DNA ▪ restriction enzymes are classified into three groups, based on how they bind and where they cut • Type II endonucleases: o they bind where they cut (most useful) o do not require ATP o cut at symmetric sites o can leave staggered ends (single-stranded regions) which can be resealed o Some leave 5’ overhangs, others 3’ overhangs; Others leave blunt ends o Restriction sites can be used as physical markers on the DNA ▪ (Size can be determined by electrophoresis.) ▪ The logic of nucleic acid sequencing by the Sanger method and 454 technology • Sanger method: o This method depends on synthesis of DNA: ▪ a primer is added that is complementary to a specific sequence on a single- stranded template to begin DNA synthesis • primer must have a free 3’-OH which permits chain elongation ▪ DNA polymerases add nucleotides (in the 5’ 3’ direction) • An altered DNA polymerase that lacks exonuclease activity (proofreading function) is used in the sanger method. ▪ All four deoxynucleotides are added in four reaction tubes ▪ One of these nucleotides is tagged (with radioactive nucleotides (32P or 35S), but more recently with fluorescent tags) • tags are attached to a portion of the nucleotide that does not participate in base pairing ▪ In each tube, a different 2’,3’-dideoxynucleotide is added • Because the dideoxynucleotides lack a 3’-OH, synthesis stops when one is incorporated ▪ There is very little of the dideoxy, but when incorporated, a chain terminates • This generates a series of oligonucleotides with different lengths, which are separated by size • The smallest fragments travel further (and fastest). • 454 technology: o Primer + DNA is immobilized on beads; beads are loaded into very small wells o DNA polymerase (DNP) + one nucleotide, wash then o DNP + a second nucleotide, wash then third and fourth in separate steps o (Up to 500 cycles of the four steps) o When a nucleotide is added light is emitted ▪ Pyrophosphate is released, and the first reaction of sulfate reduction is reversed to form ATP and sulfate ▪ ATP + luciferin emits light o o The structural differences between A-, B-, and Z-DNA. Do these occur inside cells? • Nucleic acids are inherently flexible (there are seven degrees of rotational freedom) • B-DNA: (occurs in cells) o H-bonding is only possible if strands are antiparallel o Polar sugar phosphates are on the outside o Hydrophobic bases are on the inside o in a ladder structure, water could have access between base pairs o Helical twisting brings the base pairs closer o One twist is 10 to 10.6 bp and 3.4 nm ▪ The A:T and G:C pairs have virtually the same size o stability of the structure is based on a conspiracy of: hydrogen bonds in base pairs, hydrogen bonds between the hydrophilic exterior and water, maximal separation of the phosphates (which allows their interaction with Mg++ or other cations), and hydrophobic interactions between base pairs o The antiparallel orientation means that the sugar phosphates that bind the glycosidic bonds are not exactly opposite each other in the cylinder ▪ The base pairs consequently divide the space within the cylinder unequally, and create major and minor grooves ▪ o Proteins that bind DNA read the pattern of H-bonding presented by certain base sequences in the grooves o Propellor twist is twist along an axis perpendicular to the helical axis ▪ confers additional stability by further preventing interactions with water ▪ • A-DNA: (does not occur in cells) o The pitch (one helical turn) is 2.46 nm and is 11 bp o bases are not nearly perpendicular to the helical axis, but are 19º off the perpendicular ▪ This form may never occur in cells ▪ Dehydrated DNA, and DNA:RNA hydrids may assume this conformation o Double-stranded RNAs assume the A conformation, since the 2’-OH prevents formation of the B-form • Z-DNA: (forms in vivo, not in cells) o The G residues assume the syn rather than anti conformation ▪ o To accommodate the change, C residues must flip, and it takes the sugar with it ▪ o In the next base pair, the purine is in the anti conformation o The structure requires alternating purines and pyrimidines (on one strand) o The sugar phosphate backbone zigzags with a left-handed orientation o Methylation of C allows Z-DNA formation even if purines and pyrimidines do not alternate ▪ Methyl groups protrude into the major grooves and the hydrophobic group destablizes B-form DNA o Z-DNA may form in vivo, and the methylation of C may be involved in gene expression o Hypermethylation can shut off tumor suppressor genes, causing cancer • DNA in solution: o DNA is a dynamic flexible molecule that can distort; the helix can bend o Small molecules called intercalating agents can insert between base pairs ▪ intercalating agents: cause unwinding of the helix, and a more ladder-like structure o Alternative hydrogen-bonded structures: how can cruciforms form; hoogsteen base pairs and triplexes; what are the factors that favor quadruplexes and where are they found • Cruciforms: (can form in cells) o an inverted repeat on a single strand can base pair and form a cruciform o These structures may form in cells, and may be sites for the binding of proteins • Hoogsteen base pairs: (not found in cells?) o Normal hydrogen-bonding involves interactions between two six-membered rings. However, hydrogen-bonding can also involve the five-membered ring ▪ alternate bonding creates the Hoogsteen base pairs o One form is detected in crystals of A and T o In slightly acid conditions, C can be protonated, and H-bond differently, and C can only have two H-bonds with G o These interactions leave atoms that normally H-bond free to form additional H- bonding, and allow triplex formation ▪ Base triplets formed when a purine interacts with one pyrimidine by Hoogsteen base pairing and another by Watson–Crick base pairing o o • H-DNA (H for protonation): (found in cells) o Stretches of CT can bond with a run of GA on another strand, forming a triple- stranded structure ▪ Small triple helical region and sharp DNA bend ▪ Purine rich region can interaction with two different pyrimidine rich regions (one Hoogstein and one Watson Crick) o This type of structure can regulate some eukaryotic genes by repressing expression o • DNA Quadruplex structures: (found in cells) o structures are G-rich, cyclic, and have Hoogsteen base interactions o Certains cations (K+, Na+, Ca++) favor these structures via the O6 carbonyl group o A variety of these structures can form o They are involved in: telomeres, Ig gene rearrangements, in gene regulatory regions, , and some human diseases o Factors that affect denaturation and renaturation. • Denaturation: strand separation (a function of G:C concent) o Can be caused by: changes in pH, temperature, and ionic strength o As the strands denature, the absorbance at 260 nm increases by up to 40% (hyperchromic shift) o When the bases are stacked, the potential to absorb UV light is reduced since the pi electrons in the aromatic rings are constrained ▪ Unstacking removes the constraints o The midpoint in the absorbance increase is called the melting temperature (or Tm) o Tm is a function of G:C content ▪ higher Tm higher G:C content o Ionic strength: ▪ Increasing ionic strength increases stability ▪ This results from decreasing the electrical repulsion of the phosphates ▪ DNA readily denatures in low ionic strength solutions o pH: ▪ At pH > 10, the bases are deprotonated, and H-bonding is disrupted ▪ at pH < 2.3, the bases are protonated, which disrupts H-bonding. o Small solutes that readily form H bonds also denature DNA (ex. Urea and formamide) • Renaturation: coming back together o denatured DNA will renature if the disrupting agent is removed o strands reassociate in a process called reannealing o strand must reassociate in a process called reannealing ▪ process is facilitated by a moderate temperature (just below melting), which permits rapid testing of different associations, until the right register is found ▪ rate depends on the complexity of the DNA • takes longer for larger DNA to find the right register than short DNA. More complex DNA takes longer to associate o Renaturation is a two-step process: o • The density of DNA is a function of G:C content. This can be used to separate DNA from other cellular material Nucleic acid hybridizations and DNA complexity. DNA tertiary structure: supercoils, topoisomerases, and gyrases • Supercoils: o Circular DNA forms supercoils o Normally DNA has about 10 bp per helical turn. Adding additional twists creates supercoiling o Negative supercoils: adds twists in the direction of unwinding ▪ Negative supercoiling favors strand separation o Positive supercoils: add twists in the direction of winding o o Supercoiled DNA is denser, and sediments faster during ultracentrifugation than relaxed DNA which has lost supercoiling • Linking number (L): the number of times two strands are intertwined o This is a constant for covalently closed circles o Assuming 10 bp per turn, a 400 bp duplex has an L of 40 o Linking number can be altered only by breaking and rejoining strands • Topoisomerases: o change the linking number, and are important enzymes in replication o Topoisomerase I: cuts one strand, lets the other strand pass through the break, and reseals the DNA o Topoisomerase II breaks a double strand, and lets another double strand pass through it ▪ Ex: DNA gyrase • DNA gyrase: o DNA gyrase adds negative supercoils, and is a type II topoisomerase • Negatively supercoiled DNA is found in cells o can arrange into a toroid (wrap around proteins) o important for chromosome structure and gene expression Nucleosomes and higher order structures of DNA. • Chromosome structure: o total length of DNA in a eukaryotic cell is 2 meters and must fit in a nucleus of 5 m (DNA must be condensed) • Nucleosome: The first layer of condensation is nucleosome formation o Proteins of chromatin (nucleoprotein complex) consists of histones and nonhistone proteins ▪ Histones are abundant; nonhistone proteins are not ▪ Nonhistone proteins are often regulators of gene expression ▪ Histones are small, arginine- and lysine-rich proteins that have a net positive charge. (note: most proteins are negatively charged.) o Four histones form an octameric core, which is the protein core of the nucleosome: (H2A, H2B, H3, and H4) ▪ Negatively supercoiled DNA is wrapped around the core ▪ 146 bases are wrapped around the core in 1.65 turns ▪ There are 40 to 60 bp between nucleosomes ▪ Histone H1 helps in organizing DNA into the nucleosomes and between nucleosomes • Structures with higher order organization include SMC proteins (structural maintenance of chromosomes), which are present in all forms of life Structures of tRNA and rRNA: major features. • RNA structure: o RNA can have double-stranded regions, and extensive secondary structure ▪ Bulges and loops formed in RNA when aligned sequences are not fully complementary. • tRNA secondary structure: o contain from 73-94 nucleotides o Double helical regions form, with a single-stranded hairpin o structure is called a clover leaf with four double-stranded regions o acceptor stem is where the amino acid is linked o The amino acid is added to the 3-OH of a terminal A ▪ The 3’ end of all tRNAs is CCA o The D loop contains dihydrouridine o anticodon loop contains seven unpaired bases, and three of them form the anticodon ▪ purine is always present at the 3’ end of the anticodon and is often alkylated (methylated, etc.) ▪ 5’ end of the anticodon is always a U ▪ A variable loop is after the anticodon loop o the last loop is a TψC loop (stands for pseudouridine) and is recognized by ribosomes o • tRNA tertiary structure: o This structure results from H-bonding between the D loop and the variable and TψC loops o H-bonding involves invariant residues (in the loops), and often includes unusual bases o structure is sort of L-shaped; maximizes hydrophobic interactions between stacked base pairs o • rRNA: o rRNAs have extensive base pairing (secondary structure) o 16S rRNA from an archaebacterium, an eubacterium, and an eukaryote have a similar structure but little sequence similarity ▪ Structure is important, not sequence o Features of DNA replication: semiconservative, bidirectional, role of helicases and topoisomerases, semidiscontinuous synthesis, Okazaki fragments • hereditary material (DNA) must be stable, in order to be stored and transmitted correctly • There must be mechanisms for change to account for variations and evolution • Semiconservative: one strand from the parent DNA remains as a template while a new complementary strand is synthesized • DNA Replication: o Specific base-pairing is the basis for DNA structure and DNA replication o Replication involves strand separation followed by copying of each strand o Bidirectional Replication: ▪ Replication begins at specific sites (origins of replication) • (oriC in E. coli) ▪ Replication proceeds in both directions, as determined by a labeling experiment ▪ E. coli chromosome is circular so that replication continues until each half is replicated ▪ This mode of replication involves two replication forks ▪ Discovered by radioactive thymidine experiment which captured a replicating e. coli chromosome • Radioactive thymidine was present on both sides indicating 2 active replication forks; proved bidirectional replication ▪ Bidirectional in eukaryotes and bacteria o DNA unwinding: ▪ strands must be separated to gain access to the hydrogen-bonding regions of the base pairs ▪ can be achieved either by: holding one end fixed, while one strand is wound around the other, or by introducing negative supercoils (One for each unwound helical repeat.) • For circular DNA, only the latter is possible • Replication in bacteria proceeds at 1000 nucleotides per second, which would introduce 100 positive supercoils (10 bp/helix) o Topoisomerases: ▪ DNA gyrase, a type II topoisomerase, introduces negative supercoils, at the expense of ATP hydrolysis ▪ Topoisomerases break phosphodiester bonds, and alter the linking number when unwinding DNA o Helicases: separate the strands ▪ drive the unwinding of DNA (ATP-dependent) but does not break phosphodiester bonds • Topoisomerases break phosphodiester bonds, and alter the linking number ▪ Once helicase separates the strands, SSB (single-stranded DNA-binding protein) keeps the strands separated ▪ E. coli has ten helicases, but only one (DnaB) is involved in DNA replication (don’t have to know DnaB name) o Semidiscontinuous DNA replication: leading and lagging strands ▪ Both strands are replicated as the replication fork moves ▪ One strand is read in the 3’-5’ direction, and can be used as a template to synthesize in the 5’-3’ direction • called the leading strand; can be synthesized continuously ▪ replication fork proceeds in the 5’-3’ direction on the other strand • DNA cannot be synthesized from this strand continuously • when a sufficient amount of this strand has been exposed, a fragment of this strand can be replicated in the opposite direction of the moving replication fork, ie.e, 5’-3’ • This is called the lagging strand, since its synthesis occurs after movement of the replication fork (leading strand synthesis occurs during replication fork movement) • This synthesis is discontinuous, and is done in pieces (Okazaki fragments) (1000-2000 bases in length) o Fragment synthesis requires an RNA primer ▪ Properties of DNA polymerases: the various activities, core and holoenzymes, the γ- 8rrrcomplex and its function, the clamp loader and the sliding clamp, the differences between DNP I and III and their functions. • General properties: o base-pairing rules govern synthesis o elongation in the 5’-3’ direction o all DNPs require a free 3’-OH to build on (DNPs cannot make the first phopshodiester bond) • E. coli has five DNPs: o I, II, and V function primarily in DNA repair o DNP III is the major enzyme of replication (only 10-20 copies per cell) • DNP I: o Requires the four dNTPs, a DNA template, and a primer (to provide the 3’-OH end) ▪ The primer base pairs with the template strand • The reaction generates a dNMP attached to the 3’-OH, and pyrophosphate (PPi) is released o DNP I can only synthesize about 20 bases, before it falls off ▪ The number of bases added is refered to as processivity ▪ DNP I is considered moerately processive o DNP I has 3’-5’ exonuclease activity and 5’-3’ exonuclease activity ▪ 3’-5’ exonuclease activity: removes nucloetides from the 3’ end of an elongating chain • activity is weak relative to the 5’-3’ polymerization • removes improper nucleotides, since polymerization cannot occur if the terminal base is not base-paired ▪ 5’-3’ exonuclease activity: acts on double-stranded DNA to remove distorted (mispaired) segments in front of the replication fork • This enzyme probably also removes the primer that intiates DNA synthesis • DNP III: o Is the replication enzyme, and it has ten subunits ▪ Replication proceeds at 1000 bases/second o The core enzyme is the simplest form that can polymerase DNA ▪ has three subunits (, ε, θ) • The other subunits increase DNP activity and processivity o Two core subunits combine with a γ-complex (which has five subunits) ▪ γ-complex is connected to DnaB (helicase) by two τ subunits (This is DNP III*.) o Each core subunit binds a β dimer, to create the holoenzyme o γ-complex: assembles the holoenzyme complex onto DNA ▪ It is called a clamp loader ▪ It catalyzes the ATP-dependent transfer of a pair of β subunits to each strand of DNA o β subunits are called a sliding clamp ▪ they form a closed ring around the DNA ▪ The clamp holds the core polymerase to the DNA, which accounts for the great processivity of the enzyme o τ subunit: allows release of the complex during lagging strand synthesis ▪ this subunit is activated when an Okazaki fragment synthesis is complete • This ejects the sliding clamp bound to the lagging strand ▪ The core DNP is immediately reloaded onto a new sliding clamp at the 3’ end of the next primer ▪ o • DNA replication summary: o 1. DNA gyrase and DnaB (helicase) unwind the DNA, and the strands are kept separate with SSB. o 2. Primase (DnaG) makes a primer off the lagging strand ( not necessary for the leading strand) o 3. The lagging strand is looped around, and each DNP moves in the 5’-3’ synthetic direction. o 4. As noted above, the sliding clamp is removed on the lagging strand, and reattaches as the primer for the new Okazaki fragment is found. o 5. DNP I excises the primer, replaces it with DNA, and finally DNA ligase seals the nicks. ▪ sealing requires adjacent 3’-OH and 5’-phosphates o 6. The end of DNA replication occurs at a terminus, Ter, where the two replication forks meet. It contains a 3-4 repeats of a short DNA sequence. One set of repeats stops clockwise replication, another stopds counterclockwise replication. A protein bound to this region blocks the action of DnaB, which in turn blocks forward progress of DNP III. o Tracking: ▪ It is often visualized that DNP III moves along the DNA. This is apparently not the case. DNP III is immobile, and DNA is pushed through. The polymerase is attached to membranes in bacteria, and the nuclear matrix in eukaryotes. Telomere replication: • These sequences form protective caps (1-12 kbp) at the ends of chromosomes • consist of short (5-8 bp) G-rich sequences that are tandemly repeated • Vertebrate telomeres are TTAGGG • DNP cannot make the extreme 5’ end because a primer is needed • Lagging strand synthesis at the 3’ ends of chromosomes is primed by RNA primase, leading to Okazaki fragment synthesis o The primer is removed, and a gap is created o Telomerase is an RNA-dependent DNA polymerase that restores this missing sequence (gap) • Telomerase (enzyme) contains the RNA that serves as a template o In humans, this component is 450 nucleotides long o The 3’ end of the DNA is used as a primer to add the repeats What are the causes of errors during DNA replication? • Changes in DNA sequence lead to genetically heritable changes. Most alterations are harmful. Some give an organism a selective advantage • Point mutations: are a class of alteration that result from a single base change (substitution) or insertion or deletion of one or more bases • Transition mutation: a point mutation in which the purine of a base pair is changed to the other purine; or the pyrimidine is changed to the other pyrimidine. o For example A to G, which would change an A:T base pair to a G:C base pair • Transversion: when a purine is substituted for a pyrimidine or vice versa. o For example, an A to C, which changes an A:T base pair to a C:G base pair • Point mutations often arise by errors during DNA replication: o One mechanism is that the base is in the incorrect tautomer, and therefore mispairs ▪ In these mispairings, an H-bond acceptor can tautomerize to an H-bond donor ▪ The proofreading function of DNP III usually catches these errors o A in syn base pairs with G o T:C base pair mediated by water How can you induce mutations? • Can be induced by mutagens o For example, a base analog can be incorporated into DNA, and be designed to cause mispairing • 5-bromouracil (5-BU): o is a thymine analog o It tautomerizes frequently, and base pairs frequently with G instead of A, which induces a transition • 2-aminopurine: o acts similarly to 5-BU; (note: adenine is 6-amino purine) o 2-AP occasionally base pairs with C instead of T, causing a transition • Hypoxanthine: o Hypoxanthine can arise in situ by oxidative deamination of adenine o Hypoxanthine base pairs with C, causing a transition • Mutagens can be base modifying agents: o Nitrous acid (HNO2): deaminates A and C ▪ C deamination creates uracil, which changes a C:G pair to a U:A pair and eventually to a T:A pair o Hydroxylamine: reacts with C, converting it to a derivative that base pairs with A o Alkylating agents: add methyl or ethyl groups to bases, which alter their H- bonding properties ▪ O6-methyl-G pairs with T ▪ These agents can also cause transversions ▪ can labilize the N-glycosidic linkage of G, which leaves a gap in the DNA ▪ A repair enzyme, AP endonuclease, cleaves the phosphodiester backbone, the DNA on one strand is removed, and a new nucleotide is inserted • A transversion results if a pyrimidine is inserted • Intercalating agents can cause frame shifts o These agents fit between base pairs, and doubles the distance between two base pairs o A nucleotide is inserted during replication o can cause inactivation of the whole gene because of inappropriate translation DNA repair mechanisms: know about photoreactivation, base excision repair, and nucleotide excision repair. • Photoreactivation: o Mutations can be repaired by illumination o Ex: E.coli and streptomycin resistance experiment (Sancar) • Base excision repair: o A damaged base is excised from the sugar-phosphate backbone by DNA glycosylase, creating an AP site o Then, an apurinic/ apyrimidinic endonuclease severs the DNA strand, and an excision nuclease removes the AP site and several nucleotides o DNA polymerase I and DNA ligase then repair the gap • Nucleotide excision repair: o UvrA2B tracks along DNA until damage detected o UvrB is a helicase, unwinds DNA, UvrA released, UvrC binds o UvrB makes 3’ cut from damage site, UvrC makes 5’ cut o UvrD, another helicase, removes damaged strand o UvrB remains and recruits DNP I, fills gap, ligase seals last bond o 15 proteins in human system, Sancar purified them o Loss of these enzymes results in Xeroderma pigmentosa (XP) o In bacteria: o In humans: o Be able to distinguish: Adenine guanine hypoxanthine Uracil cytosine and thymine Adenosine Guanosine inosine uridine Cytidine Thymidine adenylic acid (AMP) GMP IMP UMP CMP dAMP dGMP dIMP dUMP dTMP Underlined items are likely to be the subject of short essay question: • Strategies of nucleotide synthesis: precursors, when is ribose added, how is ribose activated and added • Purine salvage pathways: the role of PRPP • Deoxyribonucleotide synthesis and ribonucleotide reductase • Binding of substrates to catalytic site, the two regulatory sites • Features of DNA replication • Properties of DNA polymerases [Show More]
Last updated: 3 years ago
Preview 1 out of 53 pages
.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
By Prof. Goodluck 4 years ago
$14
5
Reviews( 0 )
$14.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 22, 2021
Number of pages
53
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 22, 2021
Downloads
0
Views
95