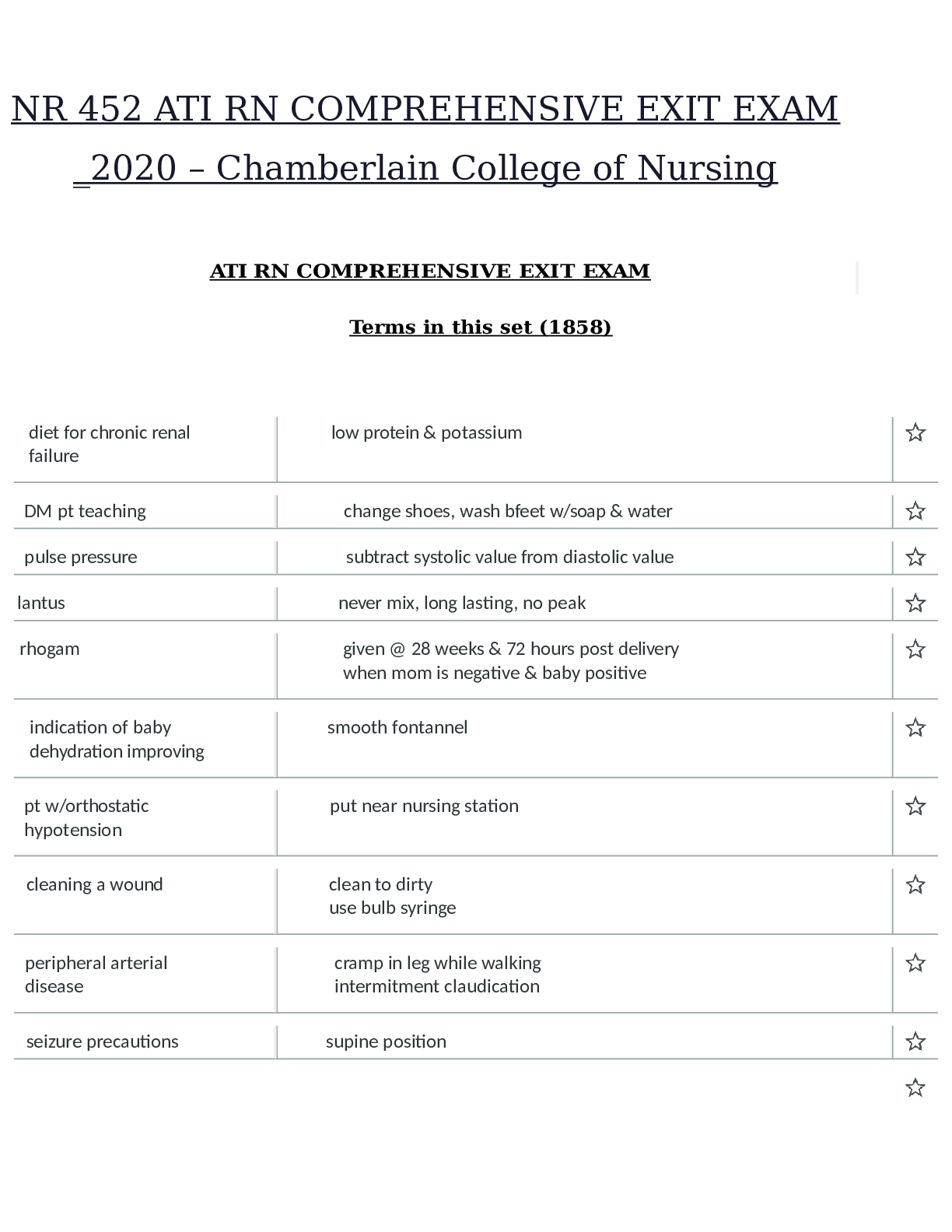
NR 452 ATI RN COMPREHENSIVE EXIT EXAM 2022
$ 55

NEEP 536: Feasibility of Controlled Fusion Energy | LECTURE PRESENTATION SLIDES
*NURSING > TEST BANKS > Sterile Product Development Formulation, Process, Quality and Regulatory Considerations 2 (All)
Part I Formulation Approaches for Sterile Products 1 Basic Principles of Sterile Product Formulation Development .......... 3 Martin A. Joyce and Leonore C. Witchey-Lakshmanan 2 Molecule and Manu ... facturability Assessment Leading to Robust Commercial Formulation for Therapeutic Proteins ........... 33 Ranjini Ramachander and Nitin Rathore 3 Polymer- and Lipid-Based Systems for Parenteral Drug Delivery..... 47 David Chen and Sara Yazdi 4 Formulation Approaches and Strategies for PEGylated Biotherapeutics .............................................................. 61 Roger H. Pak and Rory F. Finn 5 Considerations for the Development of Nasal Dosage Forms ............. 99 Jason D. Ehrick, Samir A. Shah, Charles Shaw, Vitthal S. Kulkarni, Intira Coowanitwong, Samiran De, and Julie D. Suman 6 Formulation Approaches and Strategies for Vaccines and Adjuvants .................................................................... 145 Kimberly J. Hassett, Pradyot Nandi, and Theodore W. Randolph Part II Process, Container Closure and Delivery Considerations 7 Challenges in Freeze-Thaw Processing of Bulk Protein Solutions ..... 167 Hari R. Desu and Sunil T. Narishetty 8 Best Practices for Technology Transfer of Sterile Products: Case Studies ............................................................................................. 205 Leonore C. Witchey-Lakshmanan Contents xiv 9 Transfer Across Barrier Systems: A New Source of Simplifi cation in Aseptic Fill and Finish Operations .................................................... 227 Benoît Verjans 10 Challenges and Innovation in Aseptic Filling: Case Study with the Closed Vial Technology ........................................ 249 Benoît Verjans 11 Contemporary Approaches to Development and Manufacturing of Lyophilized Parenterals ...................................................................... 275 Edward H. Trappler 12 Advances in Container Closure Integrity Testing ................................ 315 Lei Li 13 Pen and Autoinjector Drug Delivery Devices ....................................... 331 Ian Thompson and Jakob Lange Part III Regulatory and Quality Aspects 14 Particulate Matter in Sterile Parenteral Products ............................... 359 Satish K. Singh 15 Appearance Evaluation of Parenteral Pharmaceutical Products ....... 411 Erwin Freund 16 Sterile Filtration: Principles, Best Practices and New Developments ........................................................................... 431 Herb Lutz, Randy Wilkins, and Christina Carbrello 17 Intravenous Admixture Compatibility for Sterile Products: Challenges and Regulatory Guidance ................................................... 461 Manoj Sharma, Jason K. Cheung, Anita Dabbara, and Jonathan Petersen 18 Basics of Sterilization Methods .............................................................. 475 Gregory W. Hunter 19 Avoiding Common Errors During Viable Microbial Contamination Investigations ................................................................ 501 Kenneth H. Muhvich 20 Validation of Rapid Microbiological Methods (RMMs) ...................... 513 Jeanne Moldenhauer 21 Validation of Moist and Dry Heat Sterilization .................................... 535 Jeanne Moldenhauer Index ................................................................................................................. 575 [Show More]
Last updated: 2 years ago
Preview 1 out of 590 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Can't find what you want? Try our AI powered Search
Connected school, study & course
About the document
Uploaded On
Dec 16, 2021
Number of pages
590
Written in
All
This document has been written for:
Uploaded
Dec 16, 2021
Downloads
0
Views
178
Scholarfriends.com Online Platform by Browsegrades Inc. 651N South Broad St, Middletown DE. United States.
We're available through e-mail, Twitter, Facebook, and live chat.
FAQ
Questions? Leave a message!
Copyright © Scholarfriends · High quality services·