Biology > STUDY GUIDE > > A Level Biology Resources REVISION NOTES/ TOPIC QUESTIONS and PAST PAPERS (TOP SCORE) (All)
> A Level Biology Resources REVISION NOTES/ TOPIC QUESTIONS and PAST PAPERS (TOP SCORE)
Document Content and Description Below
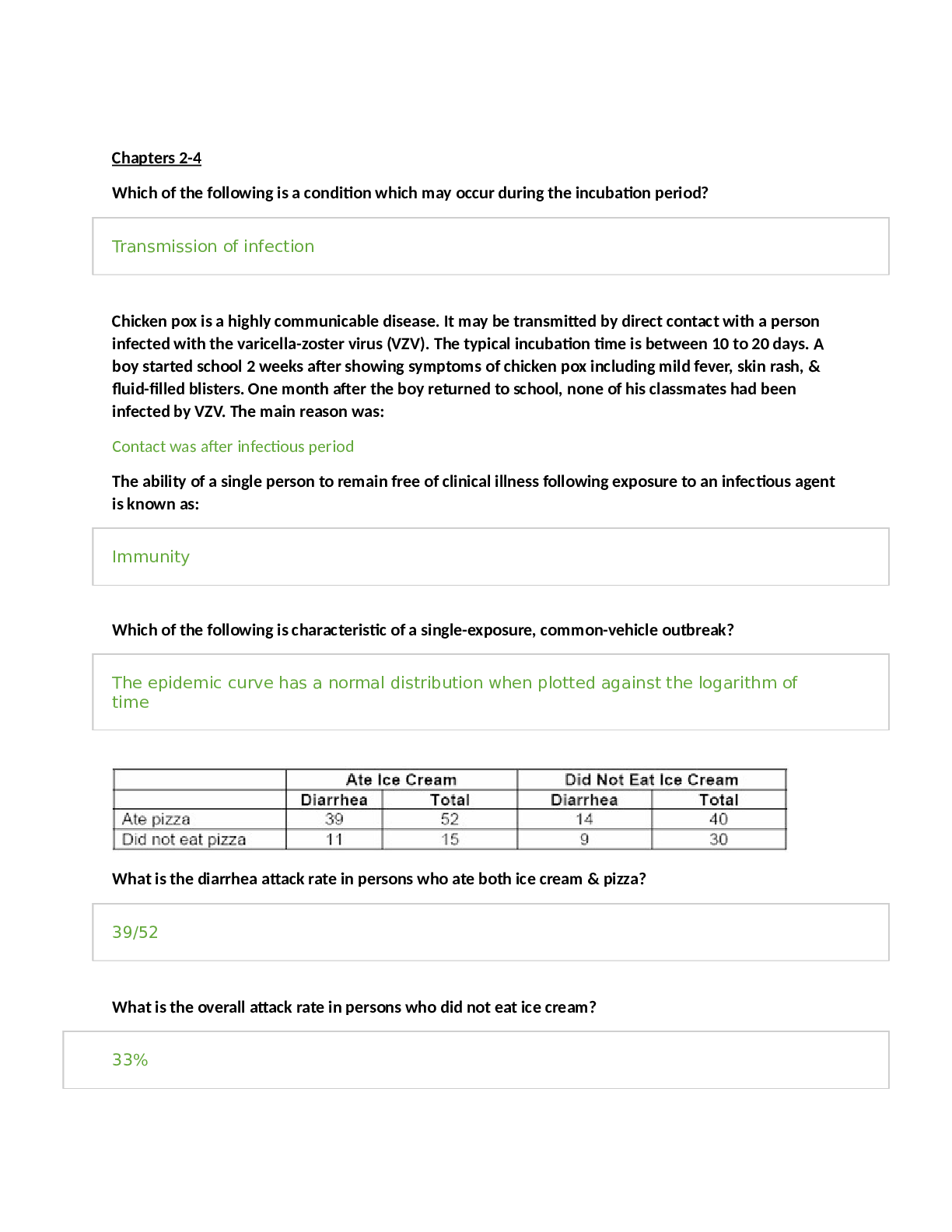
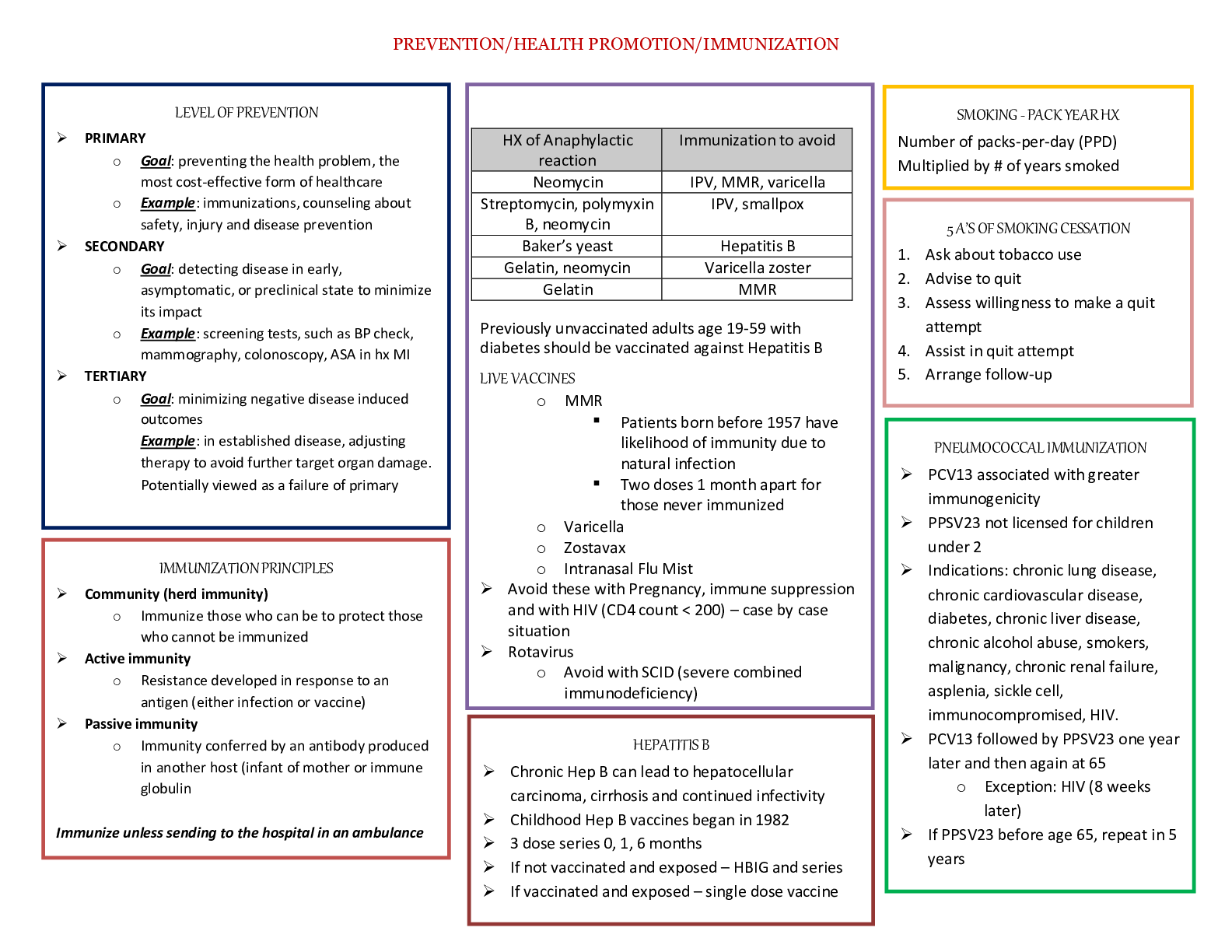
2.5 Biological Membranes YOUR NOTES 2.5.1 THE CELL SURFACE MEMBRANE Roles of Membranes Membranes are vital structures found in all cells The cell surface membrane creates an enclosed sp... ace separating the internal cell environment from the external environment Intracellular membranes (internal membranes) form compartments within the cell, such as organelles (including the nucleus, mitochondria and RER) and vacuoles Membranes not only separate different areas but also control the exchange of materials passing through them; they are partially permeable Membranes form partially permeable barriers between the cell and its environment, between cytoplasm and organelles and also within organelles Substances can cross membranes by diffusion, facilitated diffusion, osmosis and active transport Membranes play a role in cell signalling by acting as an interface for communication between cells 2.5 Biological Membranes YOUR NOTES Membranes formed from phospholipid bilayers help to compartmentalise different regions within the cell, as well as forming the cell surface membrane 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES The Fluid Mosaic Model of Membranes The fluid mosaic model of membranes was first outlined in 1972 and it explains how biological molecules are arranged to form cell membranes The fluid mosaic model also helps to explain: Passive and active movement between cells and their surroundings Cell-to-cell interactions Cell signalling The fluid mosaic model describes cell membranes as ‘fluid’ because: The phospholipids and proteins can move around via diffusion The phospholipids mainly move sideways, within their own layers The many different types of proteins interspersed throughout the bilayer move about within it (a bit like icebergs in the sea) although some may be fixed in position The fluid mosaic model describes cell membranes as ‘mosaics’ because: The scattered pattern produced by the proteins within the phospholipid bilayer looks somewhat like a mosaic when viewed from above The fluid mosaic model of membranes includes four main components: Phospholipids Cholesterol Glycoproteins and glycolipids Transport proteins Phospholipids Phospholipids form the basic structure of the membrane (the phospholipid bilayer) The tails form a hydrophobic core comprising the innermost part of both the outer and inner layer of the membrane Phospholipids bilayers act as a barrier to most water-soluble substances (the non-polar fatty acid tails prevent polar molecules or ions from passing across the membrane) This ensures water-soluble molecules such as sugars, amino acids and proteins cannot leak out of the cell and unwanted water-soluble molecules cannot get in Phospholipids can be chemically modified to act as signalling molecules by: Moving within the bilayer to activate other molecules (eg. enzymes) Being hydrolysed, which releases smaller water-soluble molecules that bind to specific receptors in the cytoplasm 2.5 Biological Membranes YOUR NOTES A phospholipid bilayer is composed of two layers of phospholipids; their hydrophobic tails facing inwards and hydrophilic heads outwards Cholesterol Cholesterol increases the fluidity of the membrane, stopping it from becoming too rigid at low temperatures (allowing cells to survive at lower temperatures) This occurs because cholesterol stops the phospholipid tails packing too closely together Interaction between cholesterol and phospholipid tails also stabilises the cell membrane at higher temperatures by stopping the membrane from becoming too fluid Cholesterol molecules bind to the hydrophobic tails of phospholipids, stabilising them and causing phospholipids to pack more closely together The impermeability of the membrane to ions is also affected by cholesterol Cholesterol increases the mechanical strength and stability of membranes (without it membranes would break down and cells burst) 2.5 Biological Membranes YOUR NOTES Glycolipids and glycoproteins Glycolipids and glycoproteins contain carbohydrate chains that exist on the surface (the periphery/extrinsically), which enables them to act as receptor molecules The glycolipids and glycoproteins bind with certain substances at the cell’s surface There are three main receptor types: Signalling receptors for hormones and neurotransmitters Receptors involved in endocytosis Receptors involved in cell adhesion and stabilisation (as the carbohydrate part can form hydrogen bonds with water molecules surrounding the cell Some glycolipids and glycoproteins act as cell markers or antigens, for cell-to-cell recognition (eg. the ABO blood group antigens are glycolipids and glycoproteins that differ slightly in their carbohydrate chains) Transport proteins Transport proteins create hydrophilic channels to allow ions and polar molecules to travel through the membrane. There are two types: Channel (pore) proteins Carrier proteins Carrier proteins change shape to transport a substance across the membrane Each transport protein is specific to a particular ion or molecule Transport proteins allow the cell to control which substances enter or leave 2.5 Biological Membranes YOUR NOTES The main components of cell membranes. The distribution of the proteins within the membrane gives a mosaic appearance and the structure of the proteins determines their position in the membrane. 2.5 Biological Membranes YOUR NOTES 2.5.2 MEMBRANE STRUCTURE & PERMEABILITY Factors Affecting Membrane Structure & Permeability The permeability of cell membranes is affected by different factors or conditions, such as: Temperature Solvent concentration Temperature Proteins and lipids (the major components in cell membranes) are both affected by temperature As temperature increases, lipids become more fluid This increased fluidity reduces the effectiveness of the cell membrane as a barrier to polar molecules, meaning polar molecules can pass through At higher temperatures, any diffusion taking place through the cell membrane will also occur at a higher speed (due to increased kinetic energy) Changes in membrane fluidity are reversible If temperatures decrease, the lipids will return to their normal levels of fluidity) At a certain temperature (often around 40°C) many proteins (including those in cell membranes) begin to denature This disrupts the membrane structure, meaning it no longer forms an effective barrier As a result, substances can pass freely through the disrupted membrane This process is irreversible Solvent concentration Organic solvents can increase cell membrane permeability as they dissolve the lipids in the membrane, causing the membrane to lose its structure Practical: Factors Affecting Membrane Structure & Permeability You can investigate how different factors affect membrane structure and permeability using beetroot Beetroot cells contain a dark purple-red pigment The higher the permeability of the beetroot cell membrane, the more of this pigment leaks out of the cell 2.5 Biological Membranes YOUR NOTES Investigating the effect of temperature on membrane permeability Apparatus Scalpel Cork borer (optional) Cutting board Ruler Test tubes Water baths Stopwatch Colorimeter (a machine that passes light through a liquid sample and measures how much of that light is absorbed) Method Using a scalpel, cut five equal-sized cubes of beetroot The pieces must have the same dimensions so that they all have equal surface areas and volumes, as these factors could affect the rate at which the pigment leaks out A cork borer can also be used, as long as the cores are cut to the same length Rinse the beetroot pieces To remove any pigment released during cutting Add the beetroot pieces to five different test tubes, each containing the same volume of water (e.g. 5cm3) Put each test tube in a water bath at a different temperature (e.g. 10℃, 20℃, 30℃, 40℃, 50℃) for the same length of time The time should be long enough to allow the pigment to diffuse into the water (e.g. around 30 minutes) Remove the beetroot pieces, leaving just the coloured liquid in the five test tubes Use a colorimeter to measure how much light is absorbed as it passes through each of the five samples of coloured liquid The higher the absorbance, the more pigment must have been released, due to a greater membrane permeability 2.5 Biological Membranes YOUR NOTES Results The general pattern you would expect to see is that as temperature increases, membrane permeability also increases As temperature increases, the phospholipids within the cell membrane move more because they have more energy Increased movement means the phospholipids are not as tightly packed together, increasing the permeability of the membrane At high temperatures, the phospholipid bilayer may even start to melt and breakdown, further increasing the permeability of the membrane In addition, the volume of water inside the cells expands, putting pressure on the membrane, causing channel and carrier proteins to deform so they can no longer control what enters and leaves the cell. These factors also increase the permeability of the membrane Temperature also affects the conformation (3D shape) of proteins as at high temperatures the intermolecular forces between amino acids are broken which affects the protein’s specificity and function If experimenting with temperatures below 0℃, membrane permeability may also be increased (once the cells have thawed again) Increased permeability can be caused by channel or carrier proteins deforming at these low temperatures Ice crystals that form can also pierce the cell membrane, making it highly permeable 2.5 Biological Membranes YOUR NOTES Example results showing the effect of temperature on membrane permeability Limitations Cuvettes are the small cuboid containers that hold the liquid to be measured in a colorimeter Cuvettes may differ in thickness (very slightly). A thicker (or scratched) cuvette will absorb slightly more light than a thinner unscratched cuvette Solution: use the same cuvette for every reading, or repeat the investigation many times and find a mean The beetroot pieces may not be identical in size and shape, meaning some test tubes could contain slightly more beetroot tissue than others Solution: cut the discs as accurately as possible using a scalpel and ruler, and repeat each investigation several times to find a mean Some parts of beetroot tissue have more pigment in their cells than others Solution: conduct several repeats, using different parts of the beetroot and find a mean 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES 2.5.3 DIFFUSION & FACILITATED DIFFUSION Diffusion & Facilitated Diffusion Diffusion is a type of transportation that occurs across the cell membrane It can be defined as: The net movement, as a result of the random motion of its molecules or ions, of a substance from a region of its higher concentration to a region of its lower concentration. The molecules or ions move down a concentration gradient The random movement is caused by the natural kinetic energy of the molecules or ions Diffusion across the cell membrane As a result of diffusion, molecules or ions tend to reach an equilibrium situation (given sufficient time), where they are evenly spread within a given volume of space The rate at which a substance diffuses across a membrane depends on several factors 2.5 Biological Membranes YOUR NOTES Diffusion Factors Table 2.5 Biological Membranes YOUR NOTES Facilitated diffusion Certain substances cannot diffuse through the phospholipid bilayer of cell membranes. These include: Large polar molecules such as glucose and amino acids Ions such as sodium ions (Na+) and chloride ions (Cl–) These substances can only cross the phospholipid bilayer with the help of certain proteins This form of diffusion is known as facilitated diffusion There are two types of proteins that enable facilitated diffusion: Channel proteins Carrier proteins They are highly specific (they only allow one type of molecule or ion to pass through) Channel proteins Channel proteins are water-filled pores They allow charged substances (eg. ions) to diffuse through the cell membrane The diffusion of these ions does not occur freely, most channel proteins are ‘gated’, meaning that part of the channel protein on the inside surface of the membrane can move in order to close or open the pore This allows the channel protein to control the exchange of ions A channel protein (open and closed) 2.5 Biological Membranes YOUR NOTES Carrier proteins Unlike channel proteins which have a fixed shape, carrier proteins can switch between two shapes This causes the binding site of the carrier protein to be open to one side of the membrane first, and then open to the other side of the membrane when the carrier protein switches shape The direction of movement of molecules diffusing across the membrane depends on their relative concentration on each side of the membrane Net diffusion of molecules or ions into or out of a cell will occur down a concentration gradient (from an area containing many of that specific molecule to an area containing less of that molecule) A carrier protein changing shape during facilitated diffusion 2.5 Biological Membranes YOUR NOTES 2.5.4 PRACTICAL: INVESTIGATING THE RATE OF DIFFUSION Practical: Investigating the Rate of Diffusion It is possible to investigate the effect of certain factors on the rate of diffusion Different apparatus can be used to do this, such as Visking tubing and cubes of agar Practical 1: Investigating the rate of diffusion using visking tubing Visking tubing (sometimes referred to as dialysis tubing) is a non-living partially permeable membrane made from cellulose Pores in this membrane are small enough to prevent the passage of large molecules (such as starch and sucrose) but allow smaller molecules (such as glucose) to pass through by diffusion This can be demonstrated by: Filling a section of Visking tubing with a mixture of starch and glucose solutions Suspending the tubing in a boiling tube of water for a set period of time Testing the water outside of the visking tubing at regular intervals for the presence of starch and glucose to monitor whether the diffusion of either substance out of the tubing has occurred The results should indicate that glucose, but not starch, diffuses out of the tubing 2.5 Biological Membranes YOUR NOTES An example of how to set up an experiment to investigate diffusion The effect of concentration gradient on the rate of diffusion can be investigated more quantitatively by: Estimating the concentration of glucose that has diffused into the water surrounding the Visking tubing at each time interval (separate boiling tubes are set up for each time interval) using the semi-quantitative Benedict’s test Comparisons of the glucose concentration between the time intervals can be made using a set of colour standards (produced by known glucose concentrations) or a colorimeter to give a more quantitative set of results A graph could be drawn showing how the rate of diffusion changes with the concentration gradient between the inside and outside of the tubing 2.5 Biological Membranes YOUR NOTES Practical 2: Investigating the rate of diffusion using agar The effect of surface area to volume ratio on the rate of diffusion can be investigated by timing the diffusion of ions through different sized cubes of agar Coloured agar is cut into cubes of the required dimensions (eg. 0.5cm x 0.5cm x 0.5cm, 1cm x 1cm x 1cm and 2cm x 2cm x 2cm) Purple agar can be created if it is made up with very dilute sodium hydroxide solution and Universal Indicator Alternatively, the agar can be made up with Universal Indicator only The cubes are then placed into boiling tubes containing a diffusion solution (such as dilute hydrochloric acid) The acid should have a higher molarity than the sodium hydroxide so that its diffusion can be monitored by a change in colour of the indicator in the agar blocks Measurements can be taken of either: The time taken for the acid to completely change the colour of the indicator in the agar blocks The distance travelled into the block by the acid (shown by the change in colour of the indicator) in a given time period (eg. 5 minutes) These times can be converted to rates (1 ÷ time taken) A graph could be drawn showing how the rate of diffusion (rate of colour change) changes with the surface area to volume ratio of the agar cubes An example of how to set up an experiment to investigate the effect of changing surface area to volume ratio on the rate of diffusion 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES 2.5.5 ACTIVE TRANSPORT Active Transport Active transport is the movement of molecules and ions through a cell membrane from a region of lower concentration to a region of higher concentration using energy from respiration Active transport requires carrier proteins (each carrier protein being specific for a particular type of molecule or ion) Although facilitated diffusion also uses carrier proteins, active transport is different as it requires energy The energy is required to make the carrier protein change shape, allowing it to transfer the molecules or ions across the cell membrane The energy required is provided by ATP (adenosine triphosphate) produced during respiration. The ATP is hydrolysed to release energy A carrier protein changing shape during active transport Active transport is important in: The reabsorption of useful molecules and ions into the blood after filtration into the kidney tubules The absorption of some products of digestion from the digestive tract The loading of sugar from the photosynthesising cells of leaves into the phloem tissue for transport around the plant The loading of inorganic ions from the soil into root hairs 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES 2.5.6 ENDOCYTOSIS & EXOCYTOSIS Endocytosis & Exocytosis The processes of diffusion, osmosis and active transport are responsible for the transport of individual molecules or ions across cell membranes However, the bulk transport of larger quantities of materials into or out of cells is also possible Examples of these larger quantities of materials that might need to cross the membrane include: Large molecules such as proteins or polysaccharides Parts of cells Whole cells eg. bacteria Bulk transport into cells = endocytosis Bulk transport out of cells = exocytosis These two processes require energy and are therefore forms of active transport Endocytosis Endocytosis is the process by which the cell surface membrane engulfs material, forming a small sac (or ‘endocytic vacuole’) around it There are two forms of endocytosis: Phagocytosis: This is the bulk intake of solid material by a cell Cells that specialise in this process are called phagocytes The vacuoles formed are called phagocytic vacuoles An example is the engulfing of bacteria by phagocytic white blood cells Pinocytosis: This is the bulk intake of liquids If the vacuole (or vesicle) that is formed is extremely small then the process is called micropinocytosis 2.5 Biological Membranes YOUR NOTES The process of phagocytosis of a bacterium by a phagocyte (white blood cell) Exocytosis Exocytosis is the process by which materials are removed from, or transported out of, cells (the reverse of endocytosis) The substances to be released (such as enzymes, hormones or cell wall building materials) are packaged into secretory vesicles formed from the Golgi body These vesicles then travel to the cell surface membrane Here they fuse with the cell membrane and release their contents outside of the cell An example is the secretion of digestive enzymes from pancreatic cells 2.5 Biological Membranes YOUR NOTES The process of exocytosis 2.5 Biological Membranes YOUR NOTES 2.5.7 OSMOSIS Osmosis All cells are surrounded by a cell membrane which is partially permeable Water can move in and out of cells by osmosis Osmosis is the diffusion of water molecules from a dilute solution to a more concentrated solution across a partially permeable membrane In doing this, water is moving down its concentration gradient The cell membrane is partially permeable which means it allows small molecules (like water) through but not larger molecules (like solute molecules) Osmosis and the partially permeable membrane. Osmosis can also be described as the net movement of water molecules from a region of higher water potential to a region of lower water potential, through a partially permeable membrane Water potential describes the tendency of water to move out of a solution. This term is used to avoid confusion between water concentration and concentration of a solution A dilute solution has a high water potential (the right-hand side of the diagram below) and a concentrated solution has a low water potential (the left-hand side of the diagram below) 2.5 Biological Membranes YOUR NOTES How osmosis works. The water moves from the region of higher water potential (dilute solution) to the region of lower water potential (concentrated solution). The water potential of pure water (without any solutes) at atmospheric pressure is 0kPa, therefore any solution that has solutes will have a water potential lower than 0kPa (it will be a negative value) 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES 2.5.8 OSMOSIS IN ANIMAL & PLANT CELLS Osmosis in Animal & Plant Cells Osmosis in animal cells Animal cells can lose and gain water as a result of osmosis As animal cells do not have a supporting cell wall (unlike plant cells), the results of this loss or gain of water on the cell are severe Animal cells losing water If an animal cell is placed in a solution with a lower water potential than the cell (such as a concentrated sucrose solution) Water will leave the cell through its partially permeable cell surface membrane by osmosis and the cell will shrink and shrivel up This is crenation (the cell has become crenated), which is usually fatal for the cell Crenation occurs when the cell is in a hypertonic environment (the solution outside of the cell has a higher solute concentration than the inside of the cell) Animal cells gaining water If an animal cell is placed in pure water or a dilute solution, water will enter the cell through its partially permeable cell surface membrane by osmosis, as the pure water or dilute solution has a higher water potential The cell will continue to gain water by osmosis until the cell membrane is stretched too far and the cell bursts (cytolysis), as it has no cell wall to withstand the increased pressure created This is fatal for the cell Lysis occurs when the cell is in a hypotonic environment (the solution outside of the cell has a lower solute concentration than the inside of the cell) This is why a constant water potential must be maintained inside the bodies of animals Animal cells in isotonic environments If an animal cell is in an isotonic environment (the solution outside of the cell has the same solute concentration as the inside of the cell) The movement of water molecules into and out of the cell occurs at the same rate (no net movement of water) and there is no change to the cells 2.5 Biological Membranes YOUR NOTES Effect of osmosis on animal cells Osmosis in plant cells Like animal cells, plants cells can also lose and gain water as a result of osmosis As plant cells have a supporting cell wall, the results of this loss or gain of water on the cell are less severe than in animal cells Plant cells losing water If a plant cell is placed in a solution with a lower water potential than the plant cell (such as a concentrated sucrose solution), water will leave the plant cell through its partially permeable cell surface membrane by osmosis As water leaves the vacuole of the plant cell, the volume of the plant cell decreases The protoplast gradually shrinks and no longer exerts pressure on the cell wall As the protoplast continues to shrink, it begins to pull away from the cell wall This process is known as plasmolysis – the plant cell is plasmolysed 2.5 Biological Membranes YOUR NOTES Plasmolysis of a plant cell that has been placed in a solution with a lower water potential than the cell itself 2.5 Biological Membranes YOUR NOTES Plant cells gaining water If a plant cell is placed in pure water or a dilute solution, water will enter the plant cell through its partially permeable cell surface membrane by osmosis, as the pure water or dilute solution has a higher water potential than the plant cell As water enters the vacuole of the plant cell, the volume of the plant cell increases The expanding protoplast (living part of the cell inside the cell wall) pushes against the cell wall and pressure builds up inside the cell – the inelastic cell wall prevents the cell from bursting The pressure created by the cell wall also stops too much water from entering and this also helps to prevent the cell from bursting When a plant cell is fully inflated with water and has become rigid and firm, it is described as fully turgid This turgidity is important for plants as the effect of all the cells in a plant being firm is to provide support and strength for the plant – making the plant stand upright with its leaves held out to catch sunlight If plants do not receive enough water the cells cannot remain rigid and firm (turgid) and the plant wilts Osmosis of water into a plant cell 2.5 Biological Membranes YOUR NOTES Comparing & Contrasting the Movement of Water by Osmosis in Plant & Animal Cells Table 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES 2.5.9 PRACTICAL: INVESTIGATING WATER POTENTIAL Practical: Investigating Water Potential Practical 1: Investigating water potential using potato cylinders It is possible to investigate the effects of immersing plant tissue in solutions of different water potentials and then use the results to estimate the water potential of the plant tissue itself The most common osmosis practical of this kind involves cutting cylinders of potato and placing them into solutions with a range of different water potentials (usually sucrose solutions of increasing concentration – at least 5 different concentrations are usually required) Method The required number of potato cylinders are cut (one for each of the solutions you are testing – or more than one per solution if you require repeats) They are all cut to the same length and, once blotted dry to remove any excess moisture, their initial mass is measured and recorded before placing into the solutions They are left in the solutions for a set amount of time (eg. 30 minutes), usually in a water bath (set at around 30o) They are then removed and dried to remove excess liquid The final length and mass of each potato cylinder is then measured and recorded 2.5 Biological Membranes YOUR NOTES 2.5 Biological Membranes YOUR NOTES You will need to use apparatus appropriately to measure out the volumes of your solutions and record your measurements Results The percentage change in mass for each potato cylinder is calculated 2.5 Biological Membranes YOUR NOTES To find the percentage change in mass, the change in mass must be divided by the initial mass and then multiplied by 100 2.5 Biological Membranes YOUR NOTES A positive percentage change in mass indicates that the potato has gained water by osmosis (net movement of water from the solution into the potato) meaning the solution had a higher water potential than the potato The gain of water makes the potato cells turgid, as the water exerts turgor pressure (or hydrostatic pressure) on the cell walls – the potatoes will feel hard A negative percentage change suggests the opposite, that is, the solution had a lower water potential than the potato The potato cylinder in the strongest sucrose concentration will have decreased in mass the most as there is the greatest concentration gradient in this tube between the potato cells (higher water potential) and the sucrose solution (lower water potential) More water molecules will move out of the potato cells by osmosis, making them flaccid and decreasing the mass of the potato cylinder – the potato cylinders will feel floppy If looked at underneath the microscope, cells from this potato cylinder might be plasmolysed, meaning the cell membrane has pulled away from the cell wall If there is a potato cylinder that has neither increased nor decreased in mass, it means there was no overall net movement of water into or out of the potato cells The solution that this particular potato cylinder was in had the same water potential as the solution found in the cytoplasm of the potato cells, so there was no concentration gradient and therefore no net movement of water into or out of the potato cells Analysis The concentration of sucrose inside the potato cylinders can be found if a graph is drawn showing how the percentage change in mass changes with the concentration of sucrose solution The point at which the line of best fit crosses the x-axis is the concentration of sucrose inside the potato cylinders 2.5 Biological Membranes YOUR NOTES A positive percentage change in mass indicates that the potato has gained water by osmosis (net movement of water from the solution into the potato) meaning the solution had a higher water potential than the potato. A negative percentage change suggests the opposite. 2.5 Biological Membranes YOUR NOTES Practical 2: Investigating water potential using onion cells Evidence of osmosis occurring in plant cells can be shown when the cells undergo plasmolysis: If a plant cell is placed in a solution with a lower water potential than the cell (such as a concentrated sucrose solution), water will leave the cell through its partially permeable cell surface membrane by osmosis As water leaves the vacuole of the plant cell, the volume of the cell decreases The protoplast (living part of the cell inside the cell wall) gradually shrinks and no longer exerts pressure on the cell wall As the protoplast continues to shrink, it begins to pull away from the cell wall This process is known as plasmolysis – the plant cell is plasmolysed This process can be observed using epidermal strips (sections of the very thin outer layer of tissue in plants) Plants with coloured sap (such as red onion bulbs, rhubarb petioles and red cabbage) make observations easier The epidermal strips are placed in a range of molarities of sucrose solution or sodium chloride solutions, of gradually decreasing water potential The strips are then viewed under a light microscope and the total number or percentage of onion cells that have undergone plasmolysis can be counted Plasmolysis may take several minutes to occur Light micrograph of normal red onion cells alongside those that have plasmolysed (artistic impression). The cells on the left are epidermal cells that have been immersed in distilled water, whilst the cells on the right are epidermal cells that have been immersed id 1.0 mol dm⁻³ sucrose solution. 2.5 Biological Membranes YOUR NOTES [Show More]
Last updated: 2 years ago
Preview 1 out of 42 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jun 14, 2022
Number of pages
42
Written in
Additional information
This document has been written for:
Uploaded
Jun 14, 2022
Downloads
0
Views
254