Course Notes > University of Louisville BIO 240 Notes
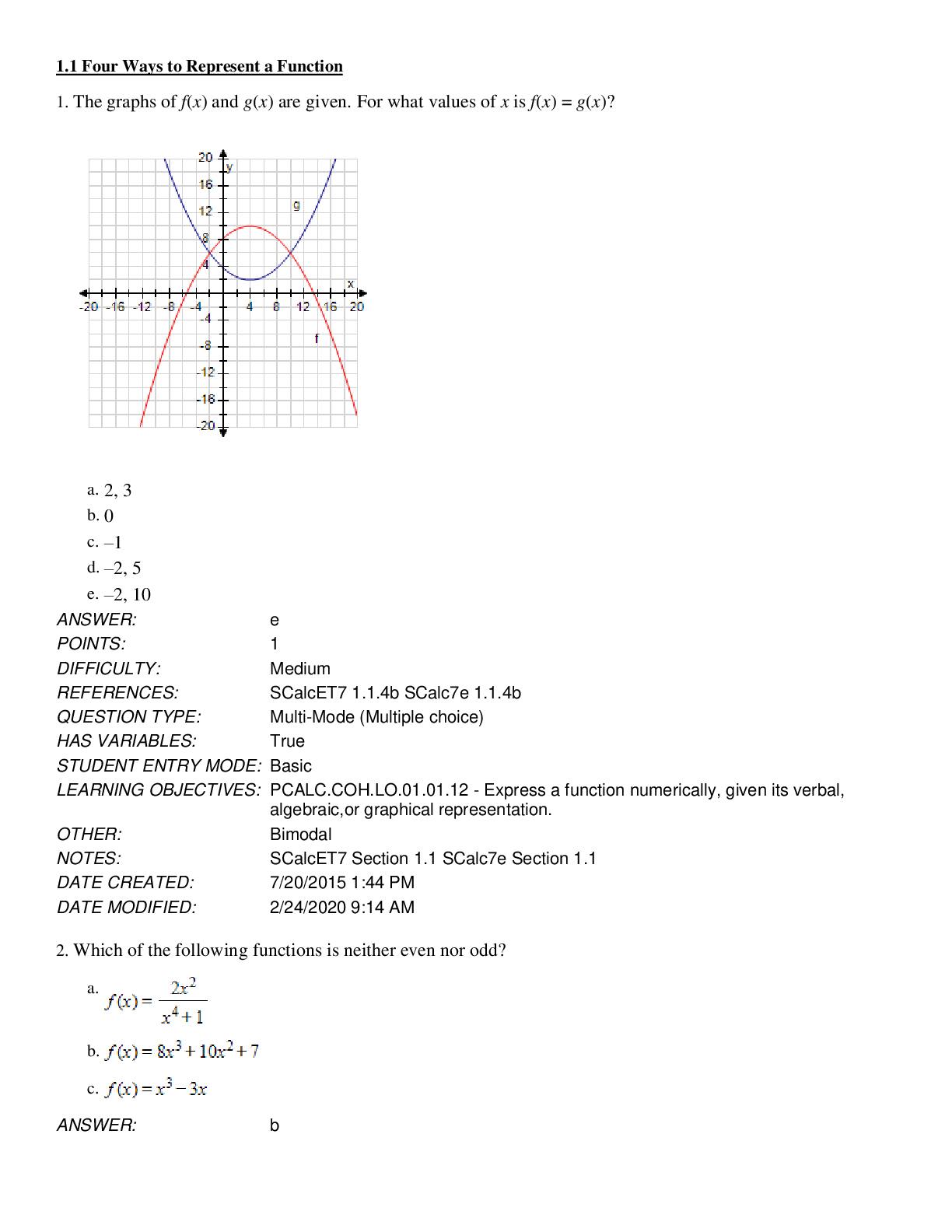
Document Content and Description Below
University of Louisville
BIO 240 BIO 240 Notes
Chapter 2: Chemical Context of Life
Bombardier Beetle ( squirts chemicals on ants)
Hydro-peroxide and hydroquinone --- defense against predators
Exa
...
mple of a Redox Reaction
Matter
Na+Cl=NaCl
Essential elements (CHONPS) 6
Trace elements – elements needed in tiny amounts but still important,
usually in enzymes
92 occurs naturally
Atom
Nucleus
Protons----determine element
Neutrons---determine isotope
Mass # = Proton + Neutrons
Atomic # = Protons
Isotopes
Same elements w/ diff # of neutrons
SAME atomic number
DIFF mass number
Isotopes important for Carbon dating (figuring out how old something
is) based on carbon isotopes
Atomic Bomb---use uranium for bombs---Iran & Uranium Enrichment
One atom has 7 protons and 7 neutrons. Another atom has 6 protons
and 7 neutrons. The two atoms are
A. Isotopes of the same elements
B. Different Elements
C. Have the same atomic number
D. A & B are correct
E. A, B & C are correct
Electrons-determine chemical behavior
1st shell---2e-
2nd shell---8e-
3rd shell---8eetc…
energy lost—towards inner shells
energy gained—towards outer shells
depends on valence electrons
Noble gases –already complete shell
An element has an atomic number of 6. How many electrons are
needed to fill the outer shell?
A. 0
B. 2
C. 4
D. 6
E. 8
Covalent bonds
-Two atoms sharing a pair of valence electrons
-pure element compounds vs molecule
- single bond
- double bond
Electronegativity
Attraction of a particular atom for the electrons of a covalent bond
Non-polar covalent bond (equal pulling)
Polar covalent bond (unequal pulling)
Ionic Bond
So unequal in valence shell attraction that electron stripped away
Bond is formed due to opposite charges
Weak Chemical Bonds
Hydrogen Bonds
What’s the advantage of a weak bond versus a strong bond?
Finite amount of energy you don’t want to use all the energy so
weak bonds are ideal for this type of reaction.
(DNA double helix is held together by hydrogen bonds, but constantly being
broken.
What type of bond would you predict between two water molecules
A. Single Bond
B. Polar Covalent Bond
C. Ionic Bond
D. Hydrogen Bond
E. A & D
January 11th 2018
-70-80% compositon of living things
-polar covalent bonds polar molecule
-Hydrogen bonds give water sit 4 properties
4 Properties
1.) Adhesion/Cohesion
a. Water falls down and gets picked up and goes against
gravity
Cohesion: Ability of water to bind to itself
Adhesion: Ability of water to bond to other objects
Hydrogen bonds allow water to go against gravity
2.) Moderation of Temperature
a.) Heat: Kenetic energy transfer
Specific Heat: heat gained/lost/ for 1g to change by 1
degree
Water has high specific heat
- Water in iron on stove
Heat breaks hydrogen bonds apart
Evaporative cooling
Transformation of liquid to gas
Evaporation surface cooling
Stabilizes our temperatures
Effect of high humidity
3.) Expansion upon freezing
a. Hydrogen bonds in ice are more ordered ice less
dense floating water
If ice sank, water would eventually freeze solid, making
life impossible
Stable hydrogen bonds in ice
4.) Water as versatile solvent
Solvent: what’s doing the absorption (water)
Solute: what’s being dissolved (sugar)
Aqueous Solution
When things go into water you get a hydration shell around them
Partial negative surrounding the Na, partially positives surrounding Cl
(when in water)
Nonionic polar molecules (sugars)
Large polar molecules with ionic/polar regions (proteins ex: oil)
NOT nonpolar molecules!
Predict how life on earth would be different if water were LESS polar?
A.)Heavier insects that water striders would be able to walk
across the surface of a pond
B.)Increased cohesion would lead to increased upward water
transport in plants
C.)Water temperature of ponds and pools would increase more
slowly when in sunlight
D.) Sweating would be less effective means of keepings
cool.
What causes an aqueous solution to have an imbalance in H+ and OH-?
When acids dissolve in water, they donate additional H+ decrease in
pH
When bases dissolve in water, they donate OH- which sop up H+ increase in
pH
The product of H+ and OH- is constant
[H+][OH-]=10^-14
pH + pOH=14
H=10-8
pH=8
What is the concentration of OH- ions in a solution where pH=10?
A. 1x10^-10
B. 1x10^10
C. 1x10^4
D. 1x10^-4
E. 1x10^7
Strong Acids & Bases
Strong acids and bases dissociate completely in water
Acid HCl: HClH+Cl- High H+
Base NaOH: NaOHNa+OH Low H+
Weak Acids & Bases are reversible reactions
Base Ammonia NH3
Acid Carbonic Acid H2CO3
Most living things cells must remain close to pH 7
Buffer
Keeps the pH where it needs to be, keeps solution relatively constant
Which of the following is buffered by carbonic acid?
A. Bone
B. Lung
C. Blood
D. Intestine
E. Skin
Carbon Compounds and Life
Aside from water, living organisms are mostly carbon-based
compounds
Inorganic: not made by living things
Organic: made by living things
Carbon can form 4 covalent bonds, why?
-Makes large complex molecules possible
Hydrocarbons
Organic molecules consisting of only carbon and hydrogen
Hydrocarbons can undergo reactions that release a large amount
of energy (gasoline)
Macromolecules
Critically important molecules of all living things
Cards, proteins, Nucleic Acids, Lipids
Macro-Large
Monomers-Small molecules to form Polymer
Lipids don’t make polymers, they don’t have monomers
Dehydration reaction: Synthesizing a polymer, water is removed and
puts 2 molecules together
Hydrolysis: Breaking down a polymer, adding H2O to solution
How many molecules of water are needed to completely hydrolyze a
polymer that is 11 molecules long?
A. 8
B. 9
C. 10
D. 11
E. 12
January 18th 2018
Diversity of the polymers
Each cell has thousands of diff macromolecules
Smalls et of monomers immense variety of polymers
Carbohydrates
Sugars & Polymers of sugars
Polymer- repeaded monomer in a sequence
Monosaccharides
Multiples of Ch2O
Glucose C6H1206
Polysaccarahde
Triose-3carbon sugar, Pentose-5 carbon sugar
Hexose-6 carbon sugar ex:–fructose & glucose
DisaccharideWHICH OF THE FOLLWING IS ONW OF THE PRIMARY CUNCTIONS OF CARBS
A. Catalyze chemical reaction
B. Energy Storage
C. Protect Cell Organelles
D. Store Cell’s Information
Lipids do not have monomeric units, therefore they do not use dehydration
reactions to form larger molecules
A. True
B. False
Lipids do not have monomeric units, therefore they do not use dehydration
reactions to form larger molecules
A. True
B. False
Sickle-cell disease is cause by a mutation in the beta hemoglobin gene that
changes a charged amino acid, glutamic acid, to valine, a hydrophobic amino
acid. This changes the shape and thus the function of the protein. Where in
the protein would you expect to find glutamic acid?
A. on the exterior surface of the protein
B. In the interior of the protein
C. Either A or B could be correct
According to the endosymbiosis theory, what is the most likely explanation
for the origin of the two mitochondrial membranes?
A. Both inner and outer membranes were derived from folding of the
prokaryotic plasma membrane
B. Both inner and outer membranes were derived from folding in of the
eukaryotic plasma membrane
C. The inner membrane was derived from folding of the prokaryotic
plasma membrane and the outer from folding in of the eukaryotic
plasma membrane
D. The inner membrane was derived from folding of the eukaryotic plasma
membrane and the outer from folding in of the prokaryotic plasma
membrane
Which of the following is a correct distinction between facilitated diffusion
and active transport?
a) active transport requires conformational changes in the transport protein,
and facilitated diffusion does not.
b) active transport requires an integral membrane protein to carry out the
transport, and facilitated diffusion does not.
c) facilitated diffusion requires a protein within the membrane, and active
transport does not.
d) facilitated diffusion depends on an existing energy gradient
acting on the transported substance, while active transport makes
such a gradient.
e) facilitated diffusion requires cellular energy (usually ATP), but active
transport does not.
A reaction has a ∆G of –5.6 kcal/mol. Which of the following is most likely to
be true?
A) The reaction could be coupled to power an endergonic reaction with a ∆G
of +8.8 kcal/mol.
B) The reaction is not spontaneous.
C) To take place, the reaction would need to couple to ATP hydrolysis.
D) The reaction would proceed by itself but might be very slow.
E) Both b & c are correct
[Show Less]
Preview 1 out of 16 pages
Purchase this document to unlock the blurred part and the rest of the document
Reviews( 0 )
Unlock this Document
Purchase the document to unlock it.
$5.00
4
1
Category: Course Notes
Number of pages: 16
Language: English
Published: 3 days ago
Downloads: 1
Views: 4
Related documents
2 Pages
Capture.JPG
University of LouisvilleBIO 240ch 13 Meiosis and Sexual Life Cycle Notes
2 Pages
Capture.JPG
University of LouisvilleBIO 240ch 9 cellular respiration and fermentation notes
4 Pages
Capture.JPG
University of LouisvilleBIO 240 Ch 7 Osmosis and Diffusion Notes
4 Pages
Capture.JPG
University of LouisvilleBIO 240ch 5 the structure and function of large molecules notes
2 Pages
111111.png
Module 9 Topic 3
About Us
We provide unclocked coursehero documents free of charge. We aim to enable the spread and access to education and information freer and easier. All you have to do is to paste the coursehero link for your desired document and click ‘UNLOCK’ You will be prompted to register for an account, if not registered already, where we shall upload the document for your access in minutes. The document will remain in your account for as long as you wish and will stay free for your access.
Quick Links
All Study Resources
Create account
Log into account
We accept
New Post
Contact Us: [email protected] +254723999996/+254727234329
Copyright © freecourseherounlocks.com. All rights reserved
twitter sharing button Tweetpinterest sharing button Pinwhatsapp sharing button Sharereddit sharing button Sharesharethis sharing button Sharefacebook sharing button Sharelinkedin sharing button Sharevk sharing button Sharearrow_left sharing button
[Show More]
Last updated: 3 years ago
Preview 1 out of 16 pages