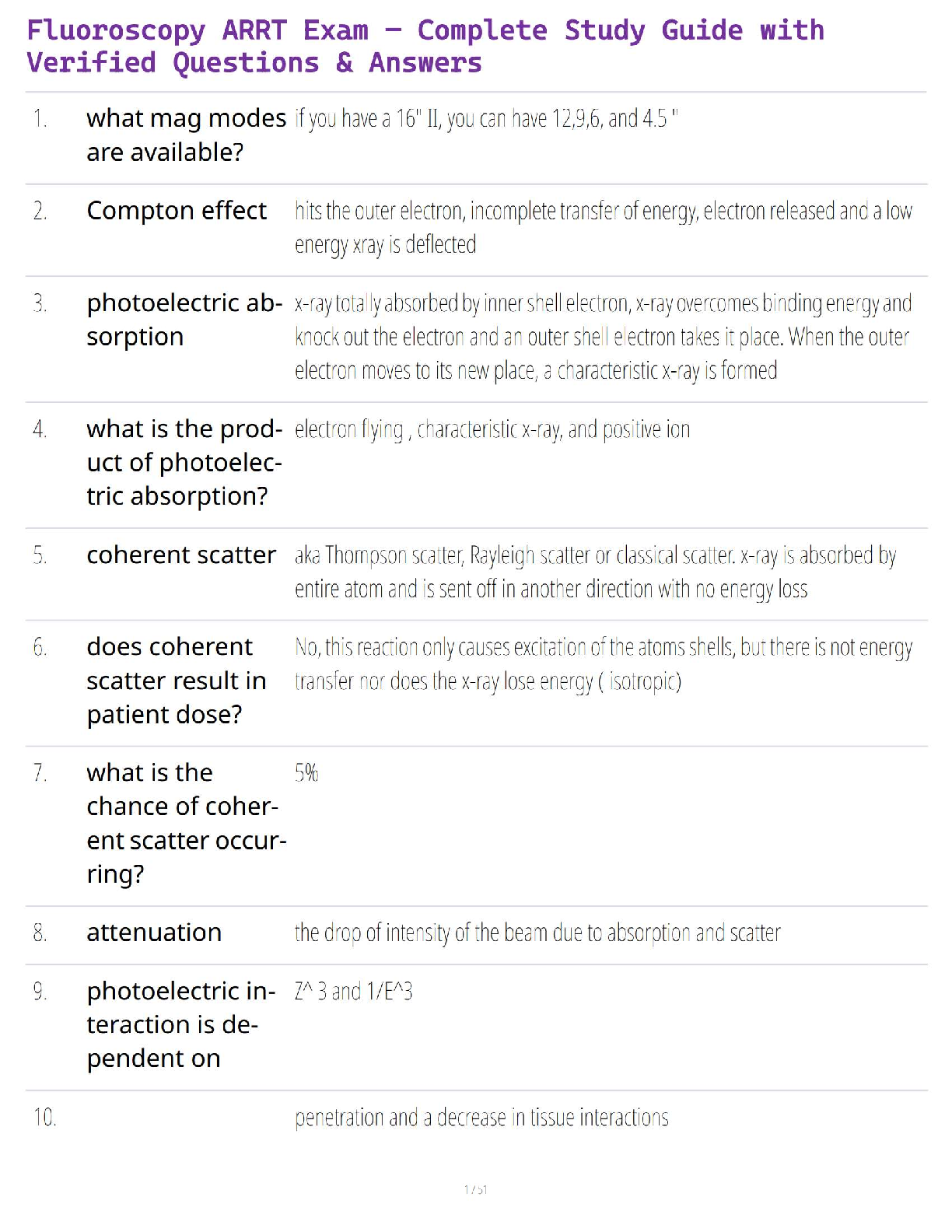
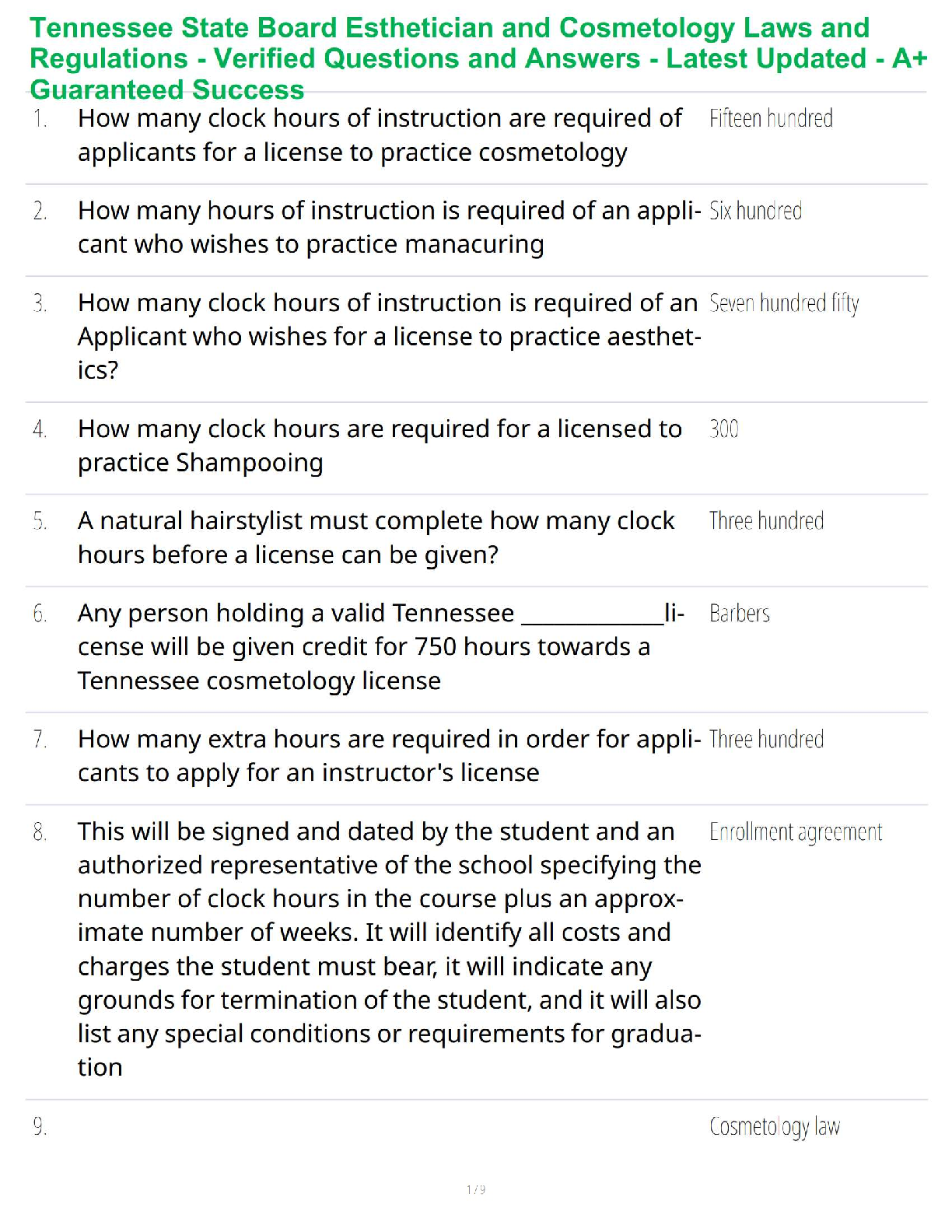
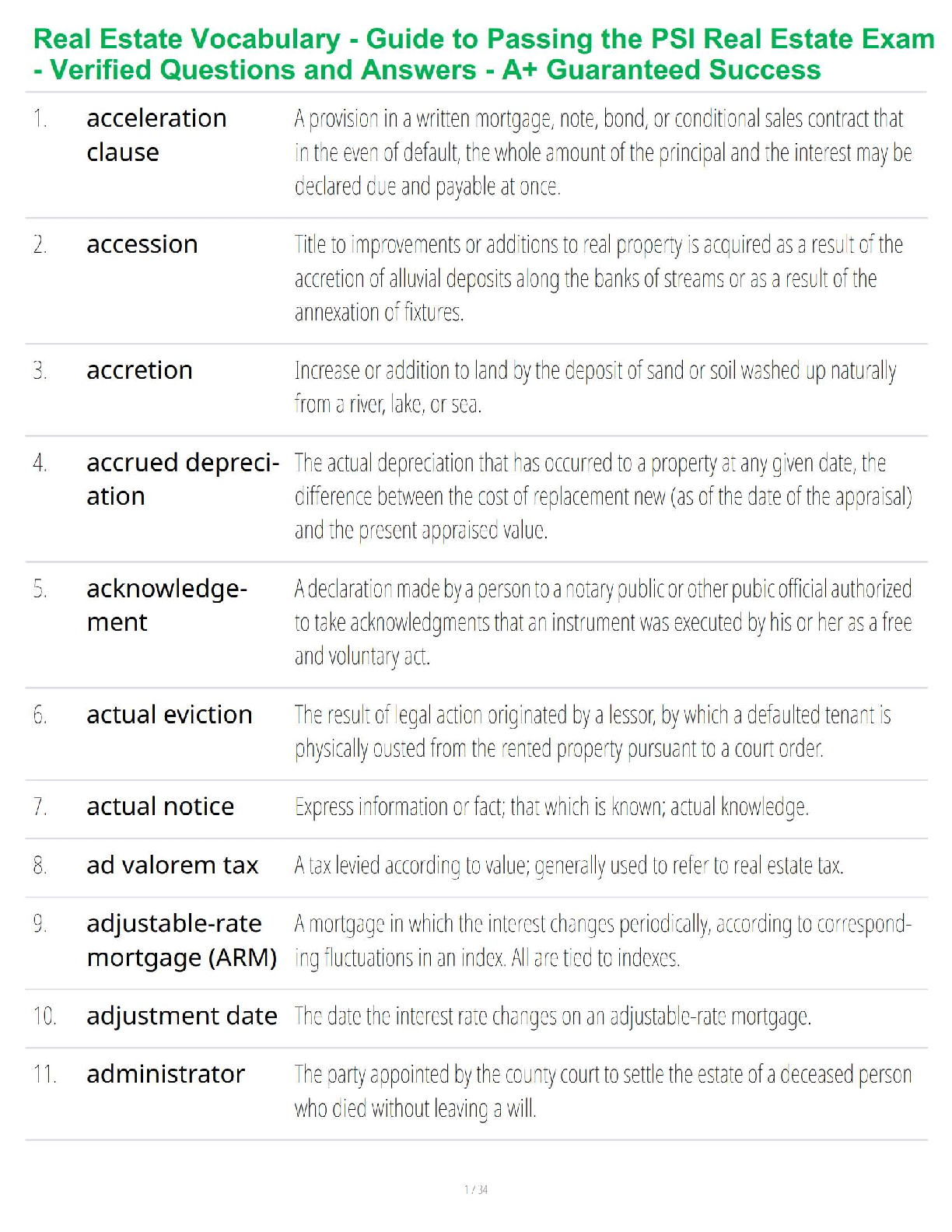
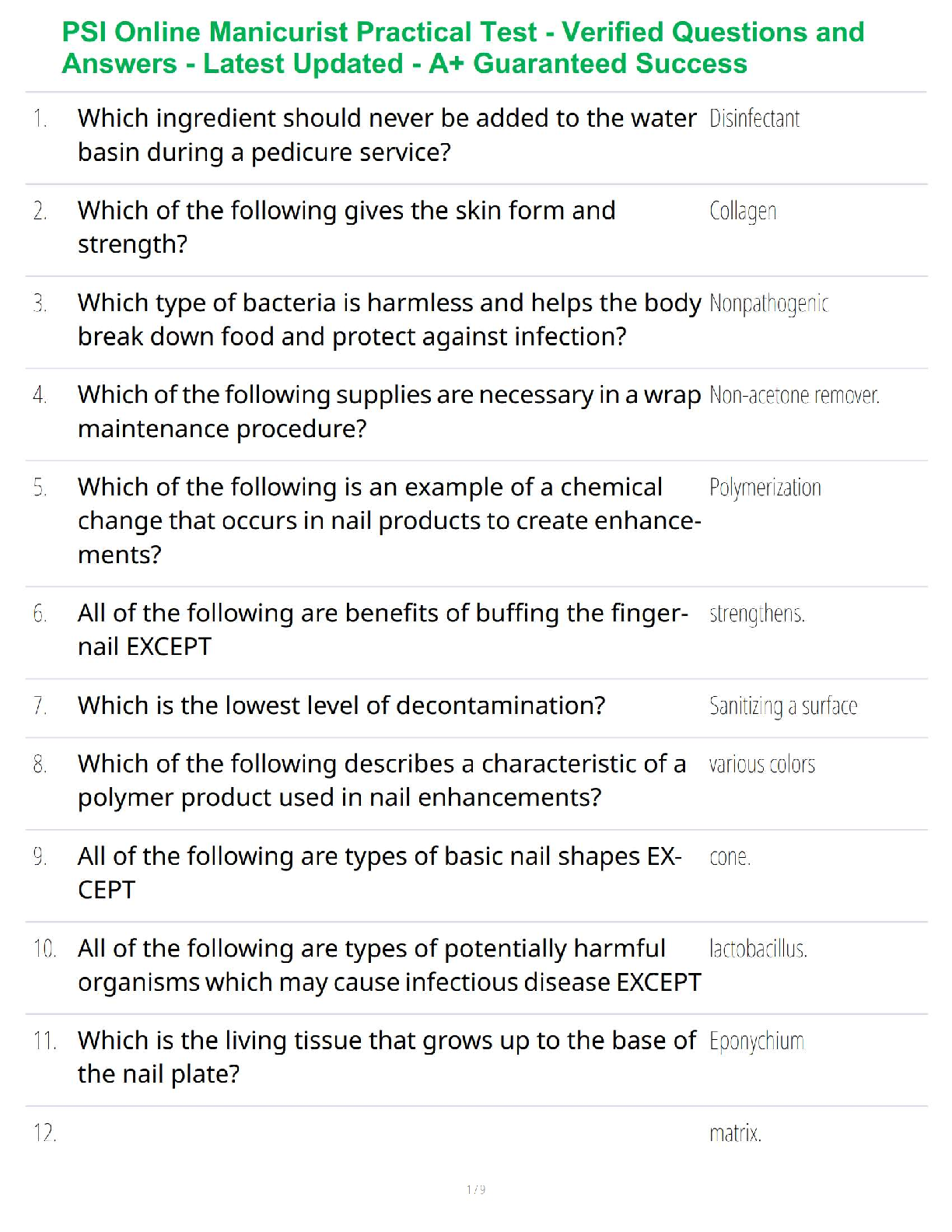
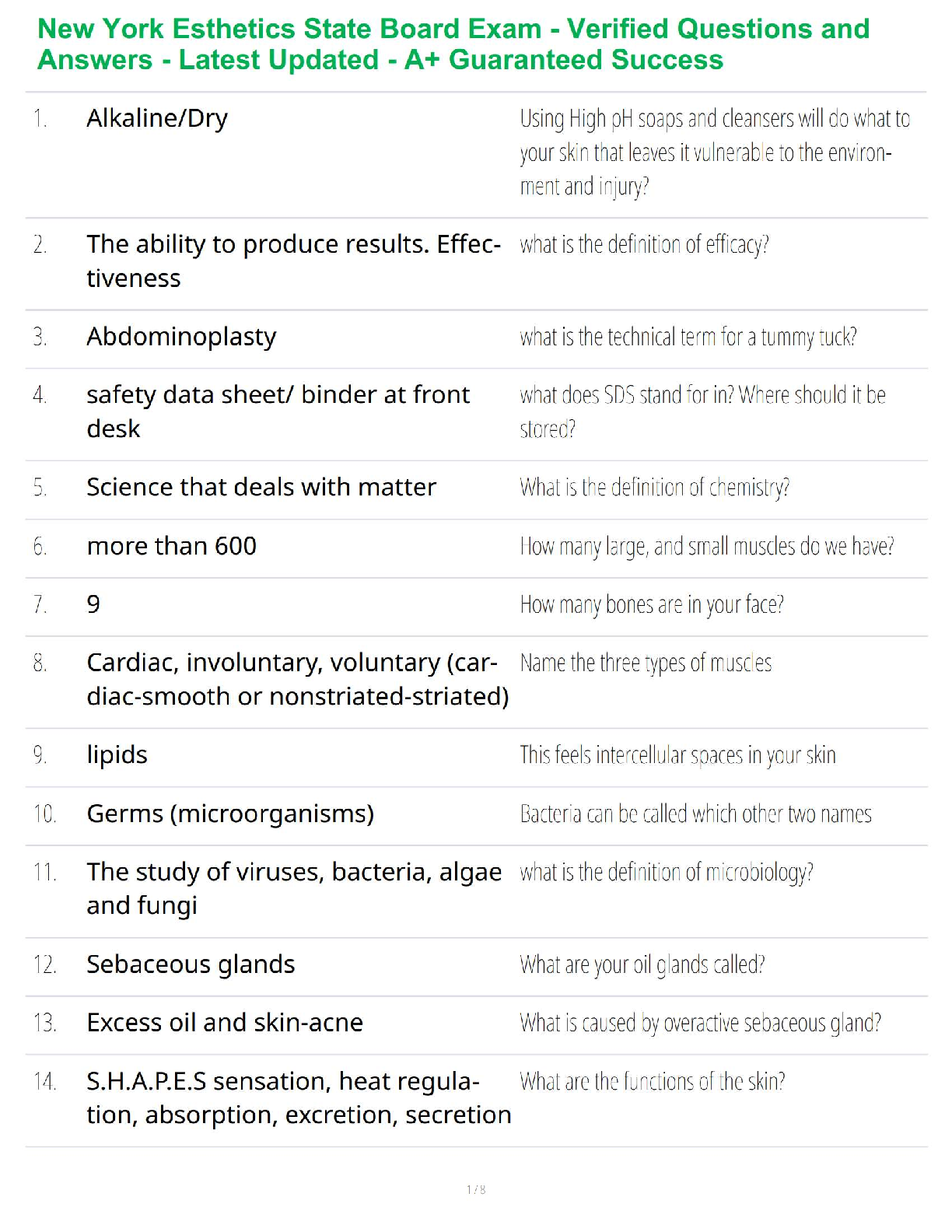
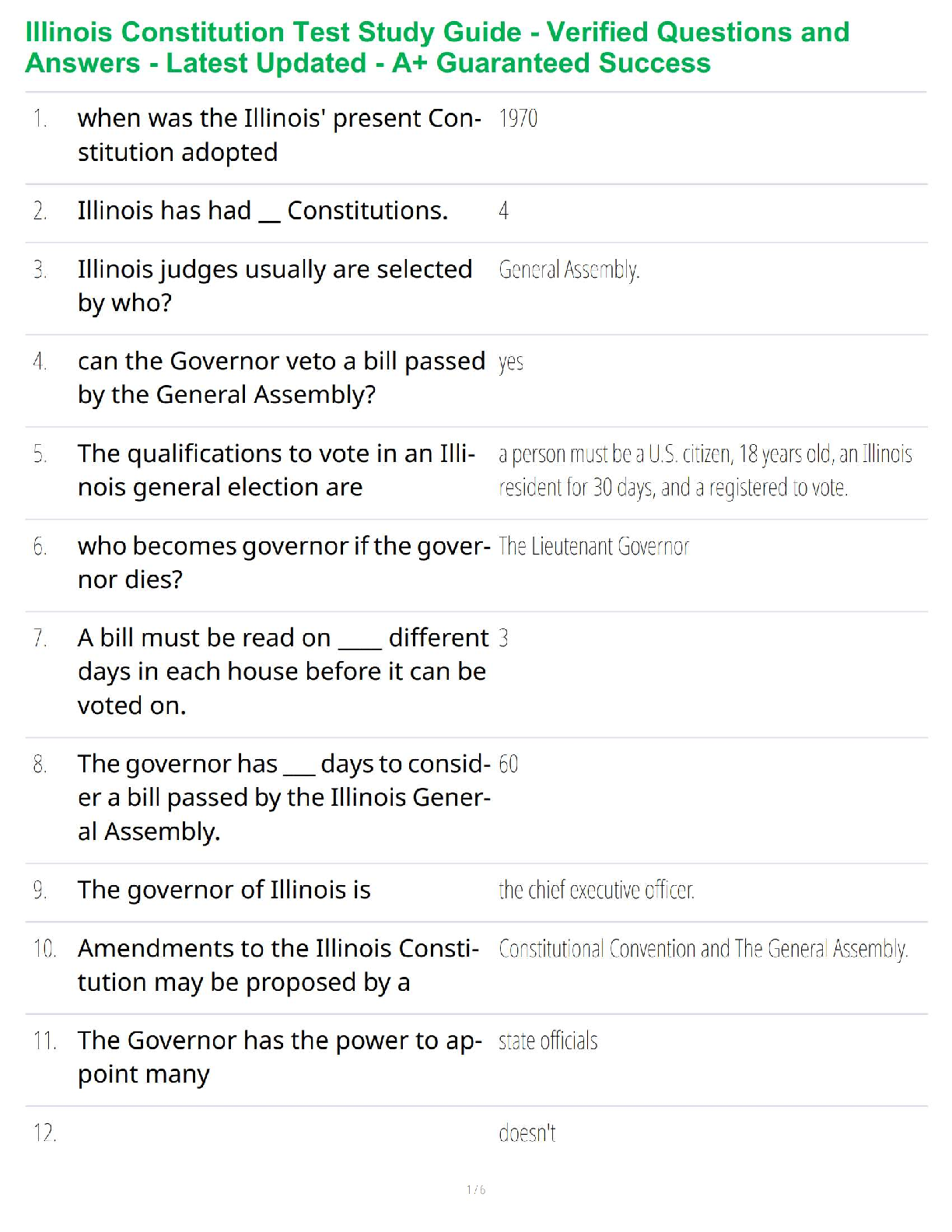
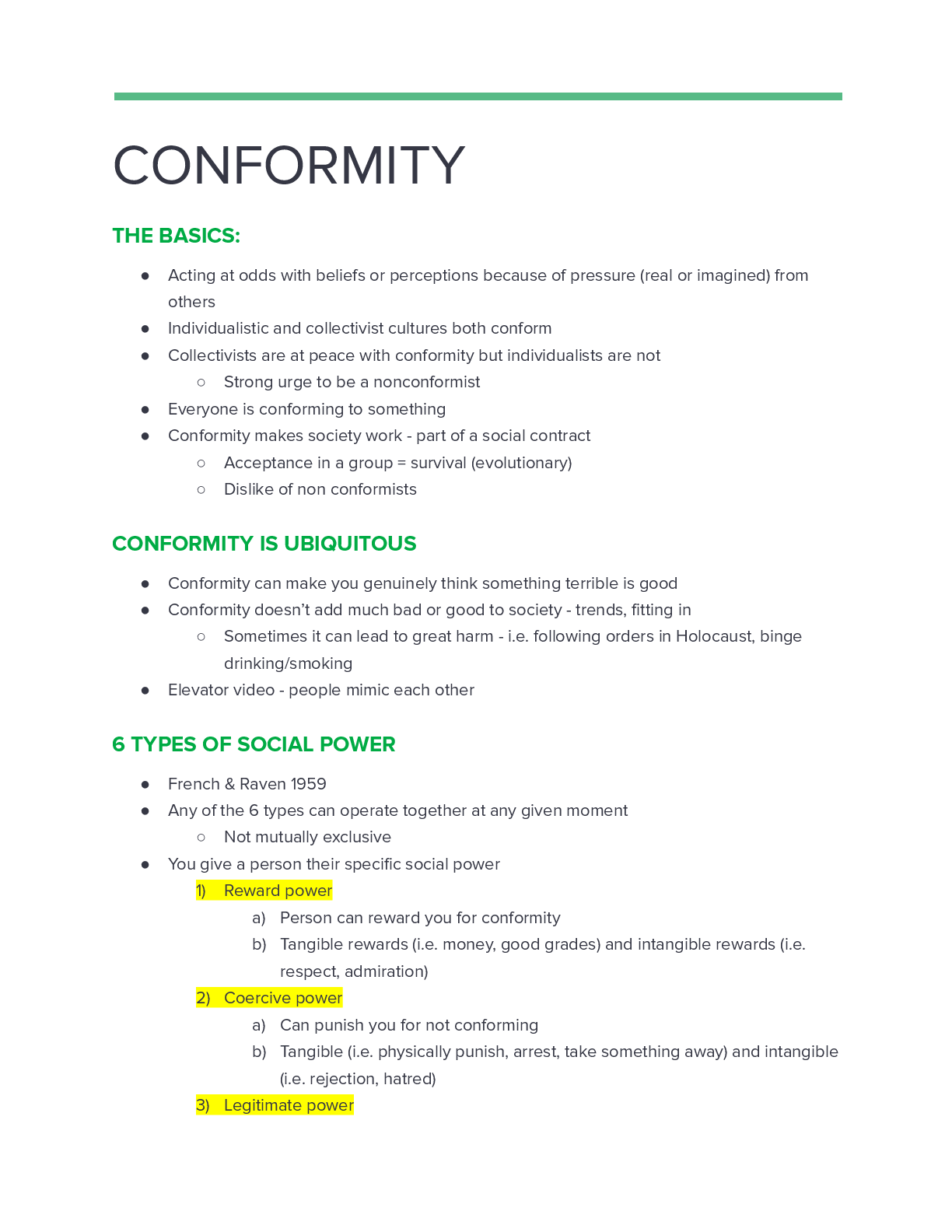
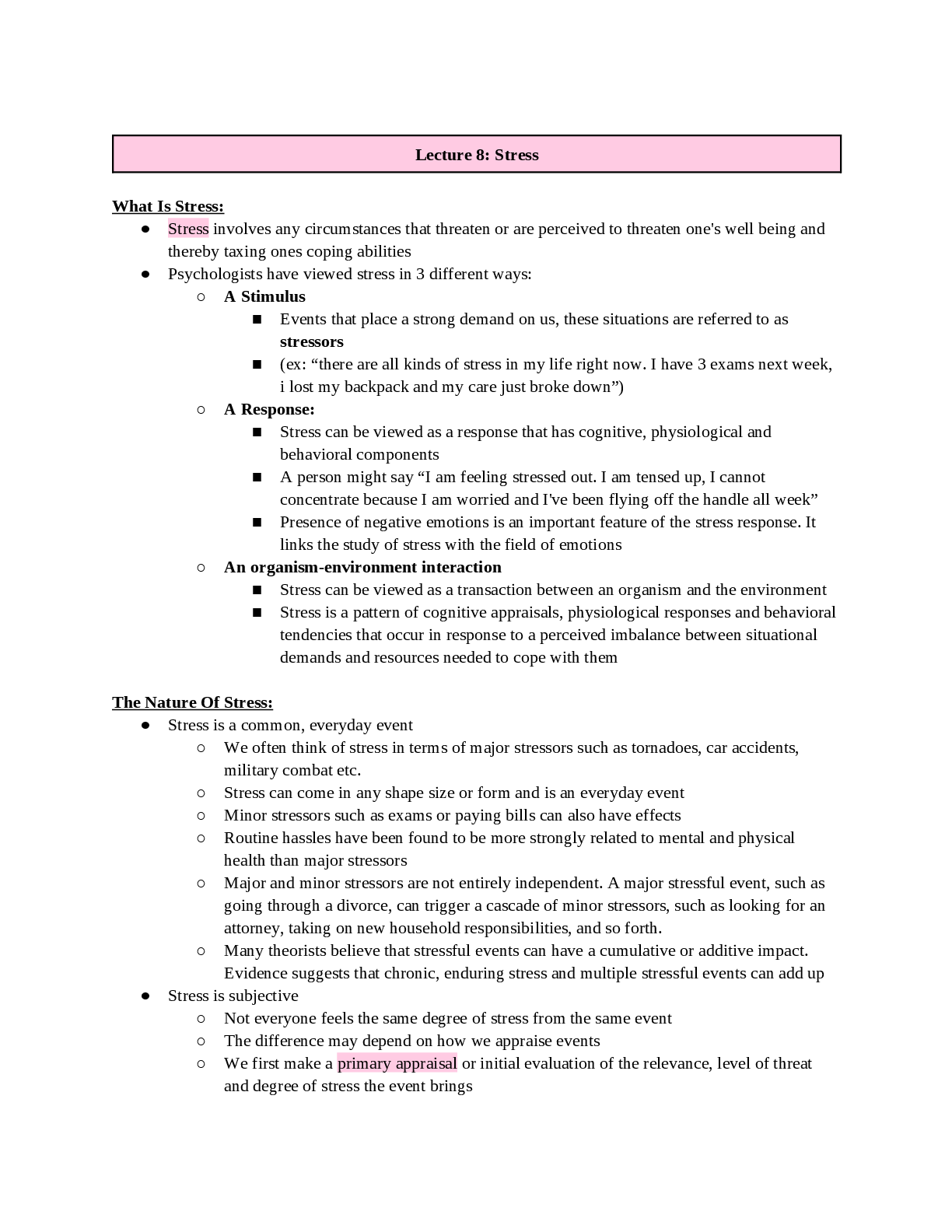
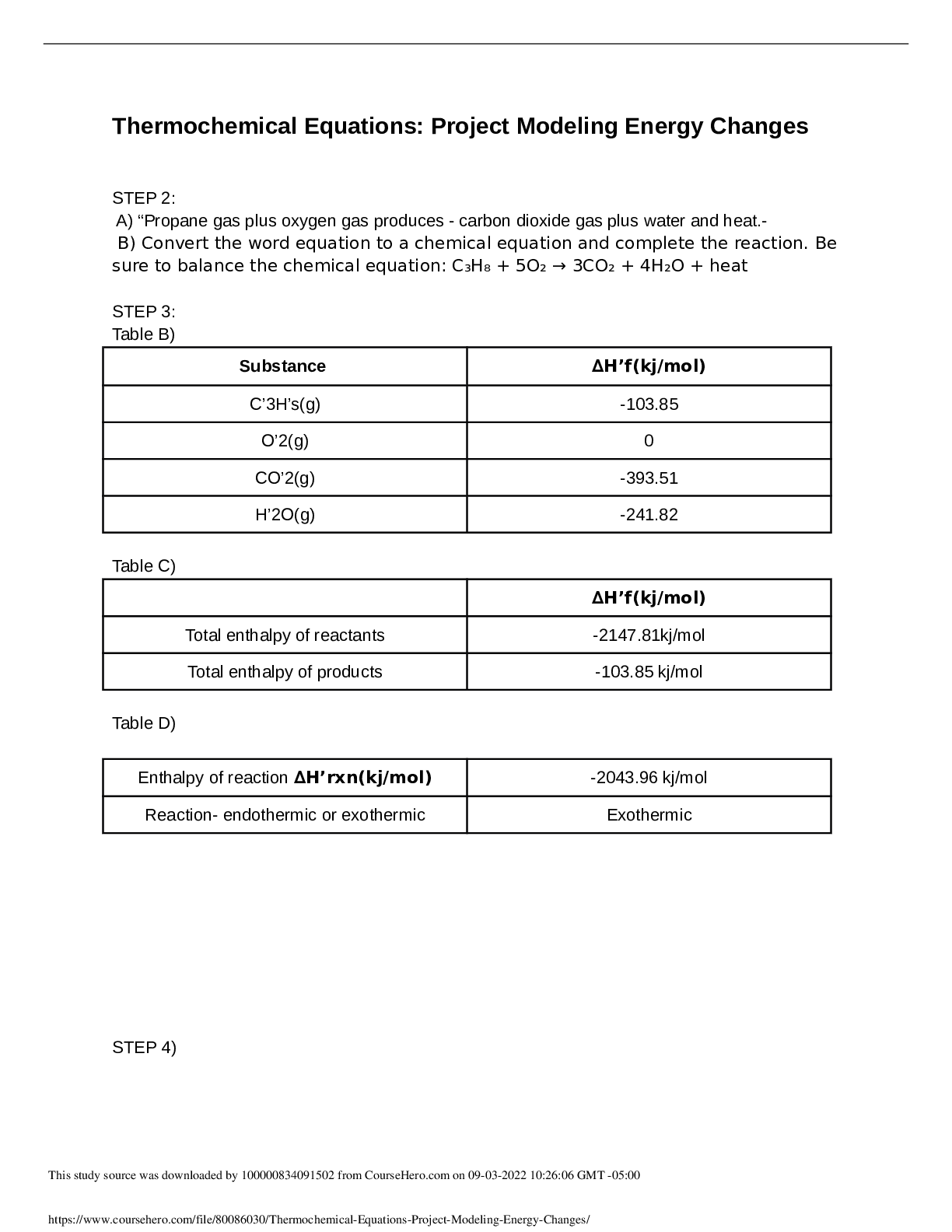
Thermochemical Equations: Project Modeling Energy ChangesSTEP 2:
A) “Propane gas plus oxygen gas produces - carbon dioxide gas plus water and heat.-
B) Convert the word equation to a chemical equation and complete the
...
Thermochemical Equations: Project Modeling Energy ChangesSTEP 2:
A) “Propane gas plus oxygen gas produces - carbon dioxide gas plus water and heat.-
B) Convert the word equation to a chemical equation and complete the reaction. Be
sure to balance the chemical equation: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O + heat
STEP 3:
Table B)
Substance ∆H’f(kj/mol)
C’3H’s(g) -103.85
O’2(g) 0
CO’2(g) -393.51
H’2O(g) -241.82
Table C)
∆H’f(kj/mol)
Total enthalpy of reactants -2147.81kj/mol
Total enthalpy of products -103.85 kj/mol
Table D)
Enthalpy of reaction ∆H’rxn(kj/mol) -2043.96 kj/mol
Reaction- endothermic or exothermic Exothermic
STEP 4)
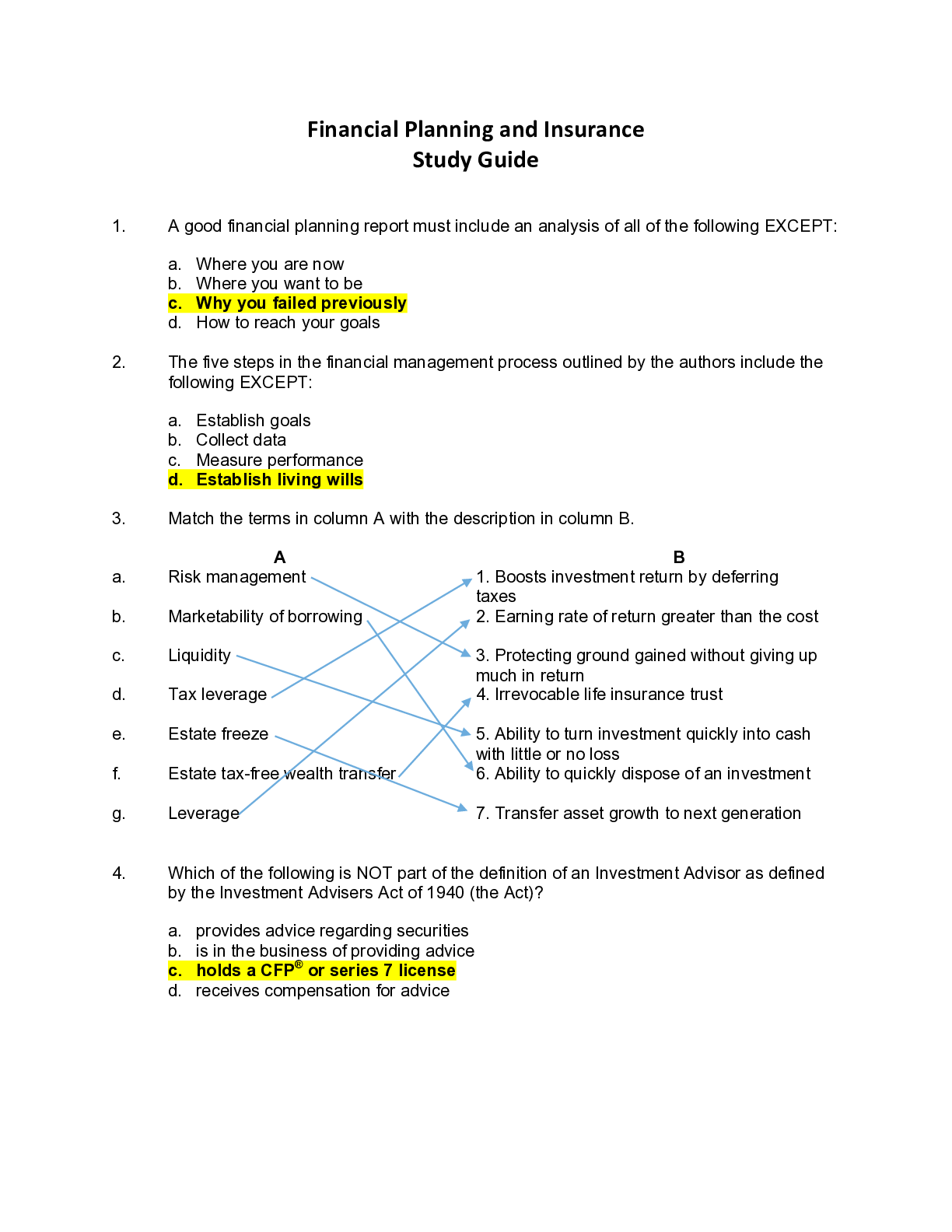
[Show More]












.png)