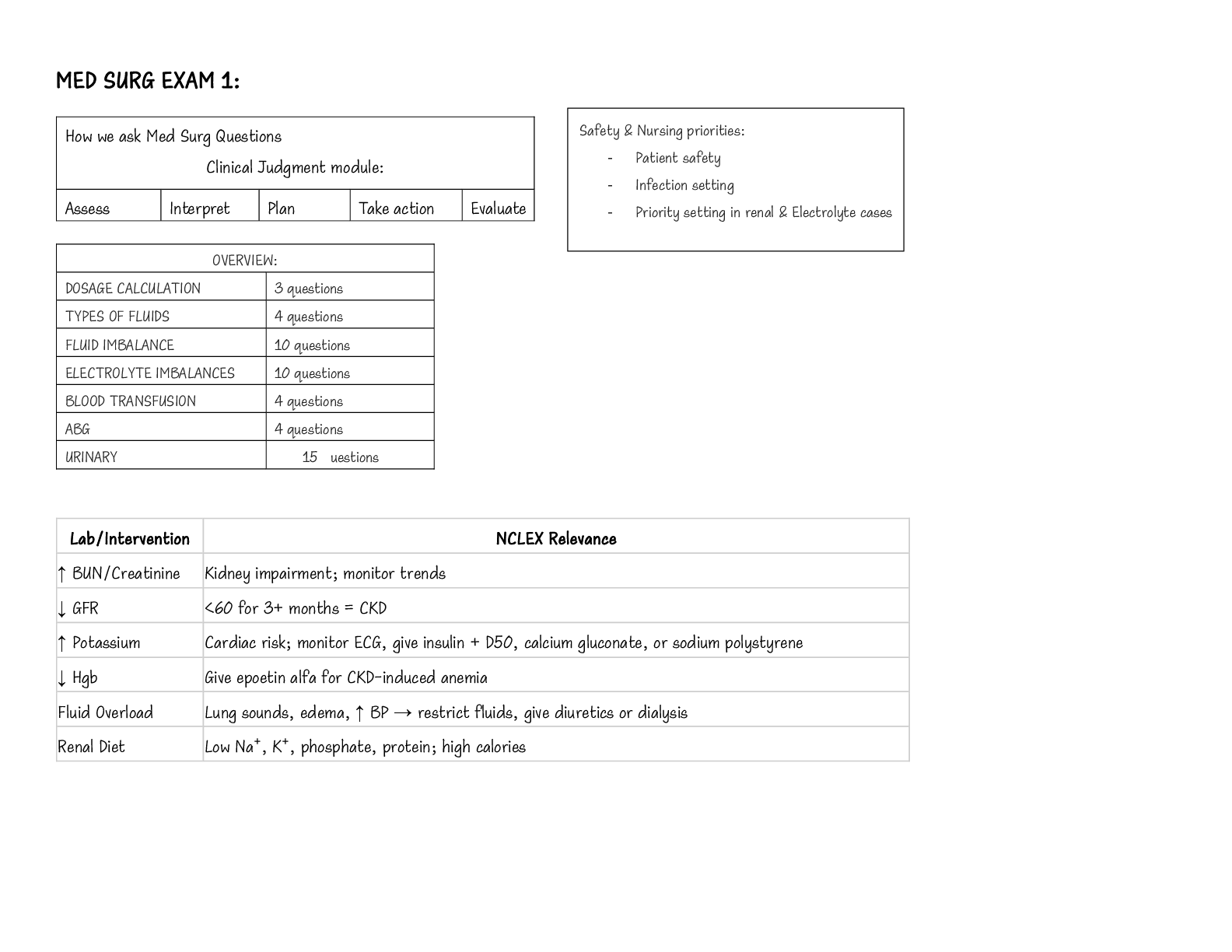
Measured Thermodynamic
Properties and
Other Basic ConceptsLearning Objectives
To demonstrate mastery of the material in Chapter 1, you should be able to:
► Define the following terms in your own words:
• Universe, s
...
Measured Thermodynamic
Properties and
Other Basic ConceptsLearning Objectives
To demonstrate mastery of the material in Chapter 1, you should be able to:
► Define the following terms in your own words:
• Universe, system, surroundings, and boundary
• Open system, closed system, and isolated system
• Thermodynamic property, extensive and intensive properties
• Thermodynamic state, state and path functions
• Thermodynamic process; adiabatic, isothermal, isobaric, and isochoric
processes
• Phase and phase equilibrium
• Macroscopic, microscopic, and molecular-length scales
• Equilibrium and steady-state
Ultimately, you need to be able to apply these concepts to formulate and
solve engineering problems.
► Relate the measured thermodynamic properties of temperature and pressure
to molecular behavior. Describe phase and chemical reaction equilibrium in
terms of dynamic molecular processes.
► Apply the state postulate and the phase rule to determine the appropriate
independent properties to constrain the state of a system that contains a
pure species.
► Given two properties, identify the phases present on a PT or a Pv phase
diagram, including solid, subcooled liquid, saturated liquid, saturated vapor,
and superheated vapor and two-phase regions. Identify the critical point and2 ► Chapter 1. Measured Thermodynamic Properties and Other Basic Concepts
triple point. Describe the difference between saturation pressure and vapor
pressure.
► Use the steam tables to identify the phase of a substance and find the value
of desired thermodynamic properties with two independent properties
specified, using linear interpolation if necessary.
► Use the ideal gas model to solve for an unknown measured property given
measured property values.
Science changes our perception of the world and contributes to an understanding of our
place in it. Engineering can be thought of as a profession that creatively applies science
to the development of processes and products to benefit humankind. Thermodynamics,
perhaps more than any other subject, interweaves both these elements, and thus its pursuit is rich with practical as well as aesthetic rewards. It embodies engineering science in
its purest form. As its name suggests, thermodynamics originally treated the conversion of
heat to motion. It was first developed in the nineteenth century to increase the efficiency
of engines—specifically, where the heat generated from the combustion of coal was converted to useful work. Toward this end, the two primary laws of thermodynamics were
postulated. However, in extending these laws through logic and mathematics, thermodynamics has evolved into an engineering science that comprises much greater breadth.
In addition to the calculation of heat effects and power requirements, thermodynamics
can be used in many other ways. For example, we will learn that thermodynamics forms
the framework whereby a relatively limited set of collected data can be efficiently used
in a wide range of calculations. We will learn that you can determine certain useful
properties of matter from measuring other properties and that you can predict the physical (phase) changes and chemical reactions that species undergo. A tribute to the wide
applicability of this subject lies in the many fields that consider thermodynamics part of
their core knowledge base. Such disciplines include biology, chemistry, physics, geology,
oceanography, materials science, and, of course, engineering.
Thermodynamics is a self-contained, logically consistent theory, resting on a few
fundamental postulates that we call laws. A law, in essence, compresses an enormous
amount of experience and knowledge into one general statement. We test our knowledge through experiment and use laws to extend our knowledge and make predictions.
The laws of thermodynamics are based on observations of nature and taken to be true on
the basis of our everyday experience. From these laws, we can derive the whole of thermodynamics using the rigor of mathematics. Thermodynamics is self-contained in the
sense that we do not need to venture outside the subject itself to develop its fundamental
structure. On one hand, by virtue of their generality, the principles of thermodynamics
constitute a powerful framework for solving a myriad of real-life engineering problems.
However, it is also important to realize the limitations of this subject. Equilibrium thermodynamics tells us nothing about the mechanisms or rates of physical or chemical processes. Thus, while the final design of a chemical or biological process requires the study
of the kinetics of chemical reactions and rates of transport, thermodynamics defines the
driving force for the process and provides us with a key tool in engineering analysis and
design.
We will pursue the study of thermodynamics from both conceptual and applied
viewpoints. The conceptual perspective enables us to construct a broad intuitive foundation that provides us the ability to address the plethora of topics that thermodynamics
► 1.1 THERMODYNAMICS1.2 Preliminary Concepts—The Language of Thermo
[Show More]















.png)