Exploration Sum: The Mole: Conversions, Mass Determination, &
Exploration Sum: The Mole: Conversions, Mass Determination, &
Hydrates
This Exploration contains targeted background content to prepare you for performing
...
Exploration Sum: The Mole: Conversions, Mass Determination, &
Exploration Sum: The Mole: Conversions, Mass Determination, &
Hydrates
This Exploration contains targeted background content to prepare you for performing the
exercises in this lesson.
Learning Objectives
Define the concept of a mole.
Distinguish between the terms: atomic mass, molecular mass, and molar mass.
Convert between mass, moles, atoms, and molecules.
Define and name hydrates by the number of moles of water held in a compound.
Explore The Mole A single atom or a single molecule is so small that
chemists are seldom able to work with one at a time. Even when weighing
the smallest quantities of substances, numerous atoms and molecules are
present. A unit of measure called a mole is used to combat this problem,
allowing for successful work with defined quantities of atoms and
molecules.
*****A mole (n) is a unit of measure, describing the amount of a chemical
substance that contains as many atoms, molecules, or formula units as
there are in exactly 12 grams of pure carbon (12C). This amount of
particles (6.022 × 1023) is referred to as Avogadro’s number.
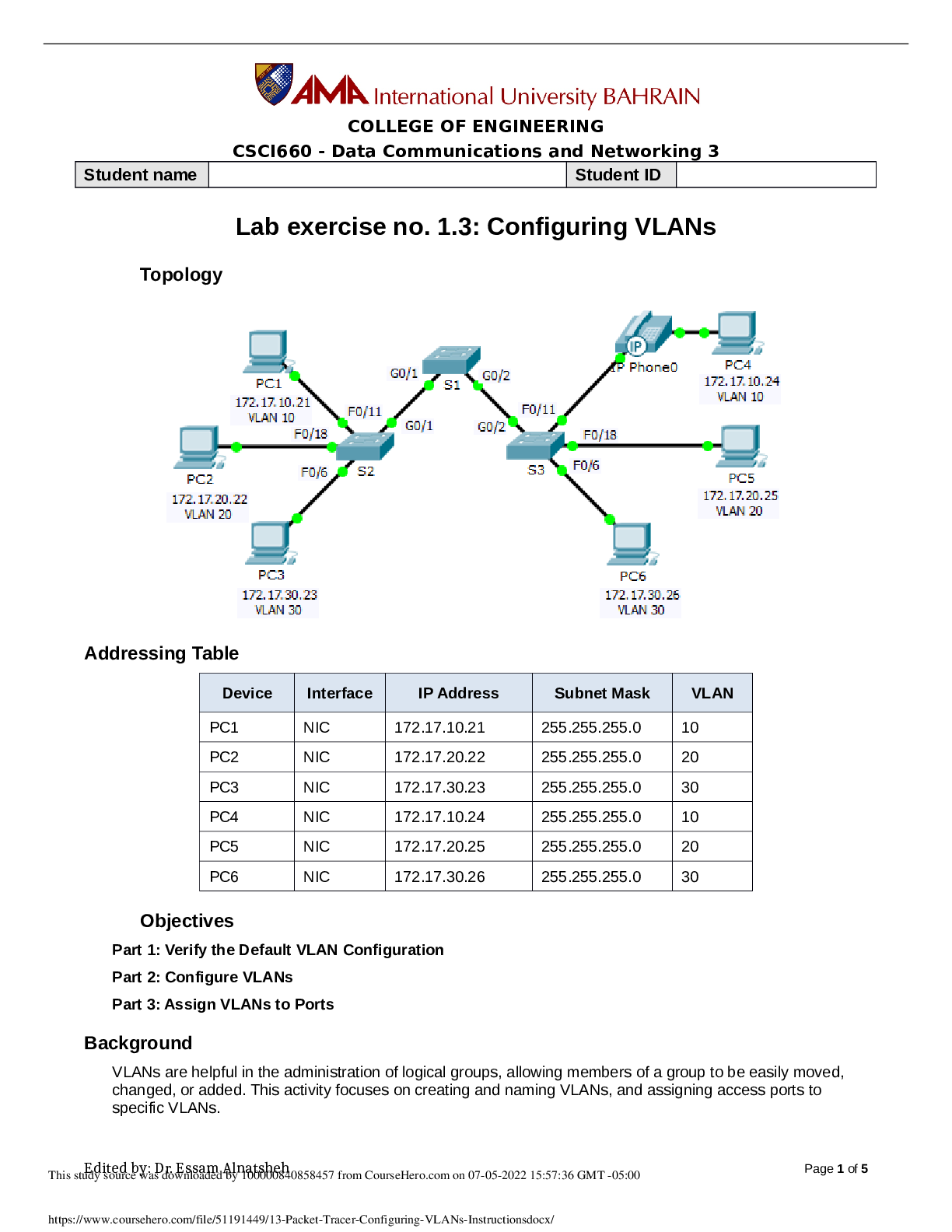
*****The atomic masses shown on the periodic table are the average
masses of the various isotopes of each element, expressed in atomic
mass units, or amu. The atomic mass unit was standardized to be equal to
exactly 1/12th the mass of one carbon-12 (12C) atom; thus, one 12C atom
has a mass of 12.000 amu. However, carbon has three isotopes (12C, 13C,
and 14C) that contain six protons each, but the isotopes vary in the
number of neutrons. The different number of neutrons leads to variation in
the amu of each isotope. Since natural carbon (C) is composed of some of
each isotope, it has an average atomic mass of 12.011 amu, as shown on
the periodic table in Figure 1.Figure 1.
Periodic Table of Elements.******The atomic mass (atomic weight) of an element is equal to
the mass in grams required to equal 1 mole of the substance.
Because atomic mass units and the mole are defined from the same
reference (carbon-12), the value on the periodic table can represent the
mass of a single atom in atomic mass units or the mass of a mole of
substance in grams. Thus, the atomic mass units shown on a periodic
table are numerically equivalent to the
****** molar masses of the elements or the mass in grams of each
element that contains one mole of atoms of that element. For example,
the element nitrogen has a molar mass of 14.007 grams, thus 1 mole of
nitrogen is equal to 14.007 grams. Likewise, the compound H2O has a
molar mass of 18.015 (H + H + O = 1.008 + 1.008 + 15.999), thus 1 mole
of H2O is equal to 18.015 grams. The mass of one mole of a substance
(molar mass) is equal to either its atomic mass expressed in grams (if it
is a monoatomic element) or its
******molecular mass expressed in grams (if it is a diatomic element or
compound).
[Show More]