BioChemistry > EXAM > BIOS 135 Week 1 Quiz (Multiple Choice & Short Questions) | 100% CORRECT ANSWERS | DeVry University (All)
BIOS 135 Week 1 Quiz (Multiple Choice & Short Questions) | 100% CORRECT ANSWERS | DeVry University
Document Content and Description Below
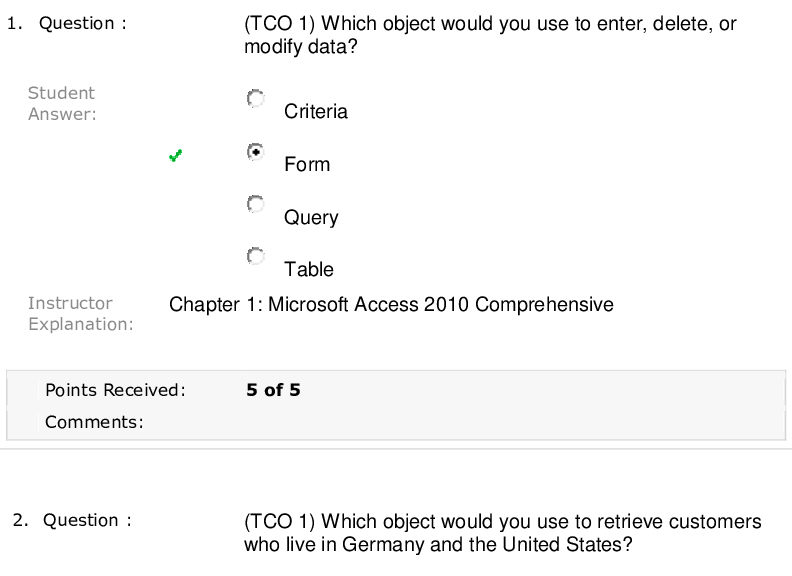
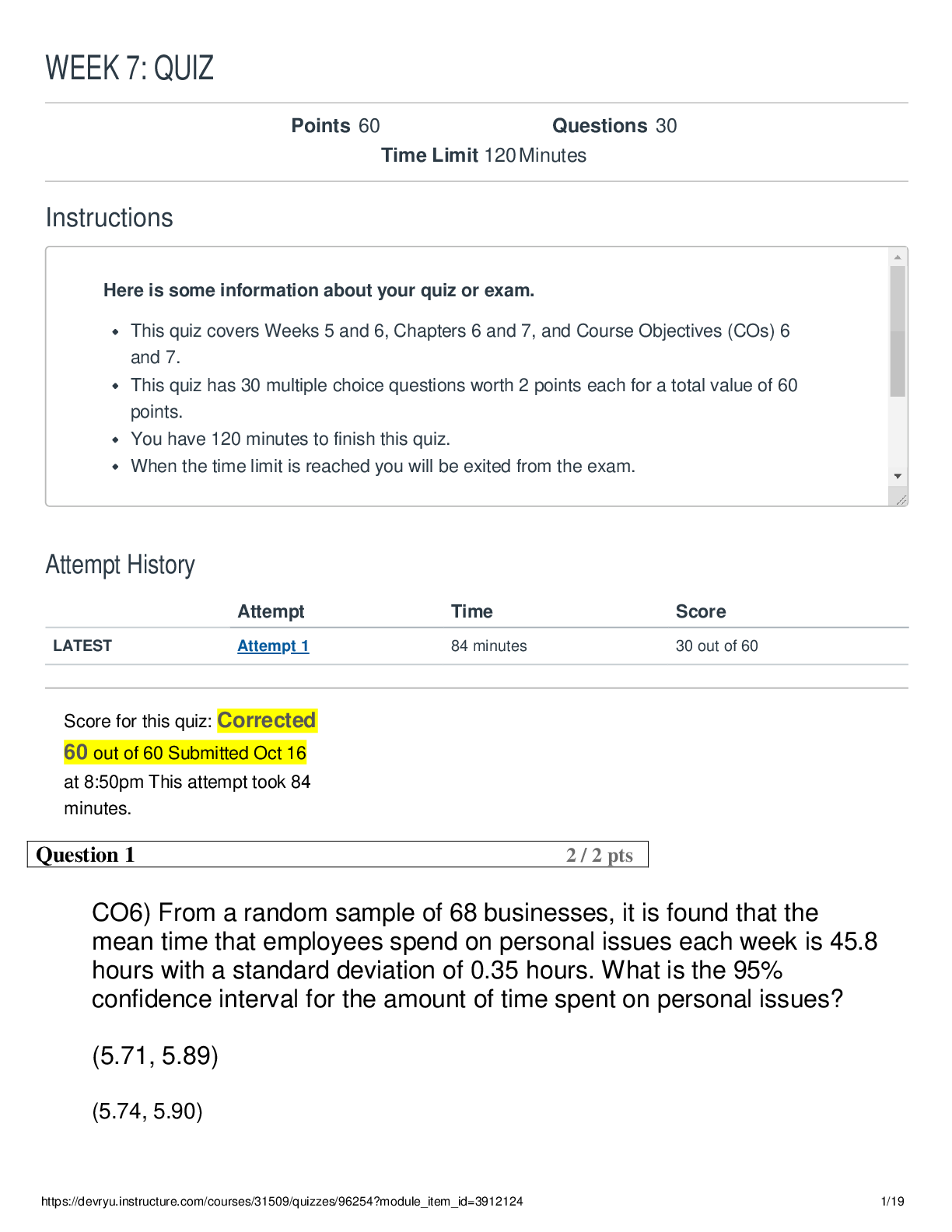
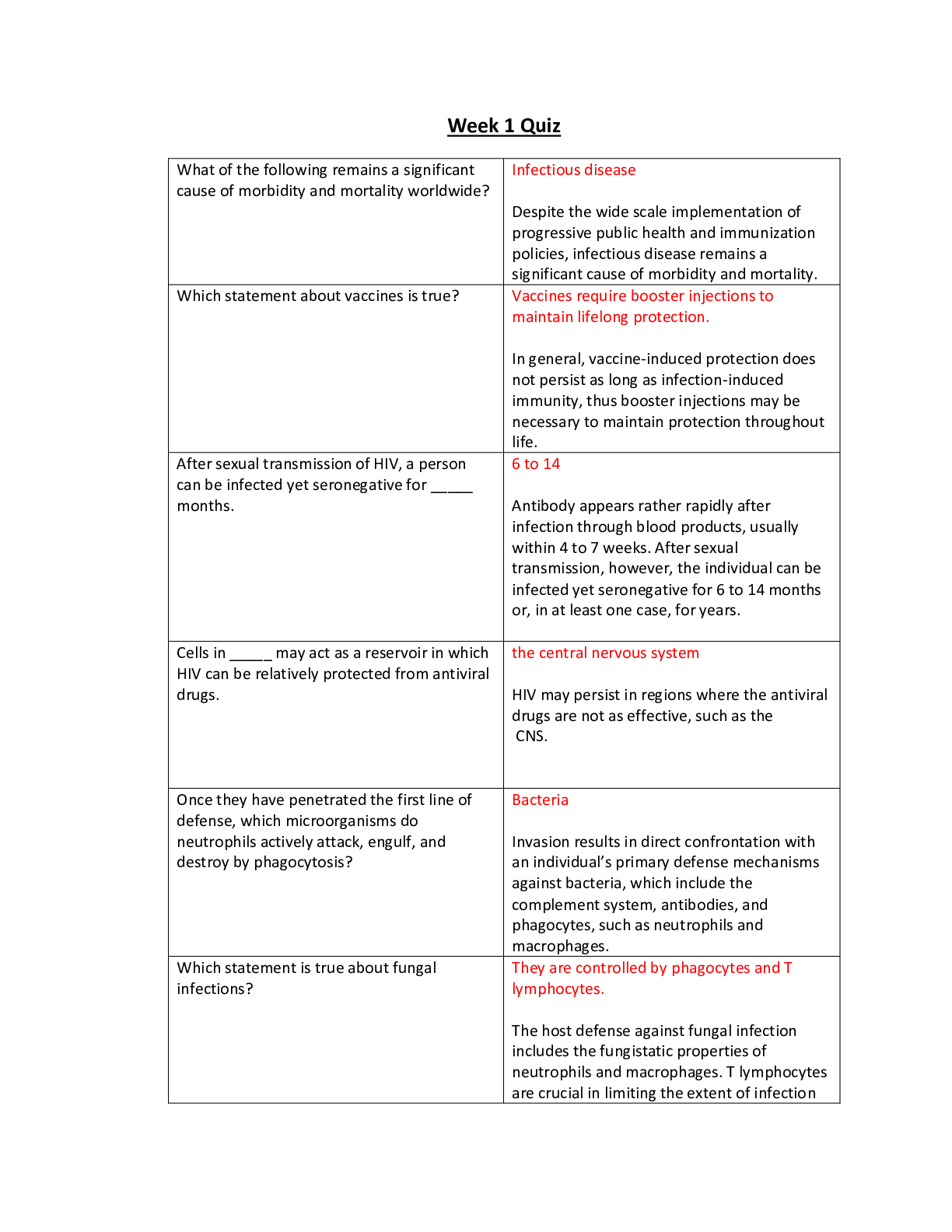
Question 1 (TCO 1) My dog will eat more of her food when I pour bacon grease over the food before I give it to her. This is an example of a(n) observation. hypothesis. experiment. theory. All ... of the above See Chapter 1. The example above is a hypothesis. It was based on an observation of increased food consumption after tasty bacon grease is added. Question 2 (TCO 1) Deductive reasoning is used to make based on a hypothesis. data theories statistical tests correlations predictions See Chapter 1. Question 3 (TCO 2) A 4 oz boneless, skinless chicken breast contains 25 g of protein. How many kcal are obtained from the protein in the chicken? 225 kcal 4 kcal 100 kcal 25 kcal 10.0 kcal See Chapter 2. 25 g (4 kcal/g) = 100 kcal. Question 4 DeVry University, May 7, 2018 (TCO 2) The formation of a liquid from a solid is known as evaporation. deposition. freezing. melting. sublimation. See Chapter 2. Solids melt to form liquids. Question 5 (TCO 2) Based on the specific heat, which type of cookware would heat up the fastest? Titanium: Specific heat is 0.5226 J/g C. Aluminum: Specific heat is 0.925 J/g C. Tin: Specific heat is 0.2274 J/g C. Copper: Specific heat is 0.3845 J/g C. Iron: Specific heat is 0.4494 J/g C. See Chapter 2. Low specific heat provides better heat transfer from heat source to pan. Question 6 (TCO 2) Which amount of heat is released when 55.0 g of water at 0 °C is frozen to ice? 0.69 cal 4,400 cal 0 cal 4.4 cal 80 cal See Chapter 2. 55.0 g ice 80 cal/g = 4,400 cal. DeVry University, May 7, 2018 Question 7 (TCO 2) How do you find the mass number of an atom? Add up the number of protons, electrons, and neutrons in the atom. Add up the number of protons and neutrons in the atom. It is equal to the number of protons in the atom. It is equal to the number of neutrons in the atom. Find the net electrical charge of the atom. See Chapter 3. Question 8 (TCO 2) Which of the following is not an example of a pure substance? NaCl Water Black coffee Aluminum foil Oxygen See Chapter 3. Question 9 (TCO 2) Which of the following statements about the periodic table is correct? A group is a horizontal row on the periodic table. A period is a column on the periodic table. The elements in each group have similar chemical properties. The B groups contain the representative elements. The A groups contain the transition elements. See Chapter 3. DeVry University, May 7, 2018 Question 10 (TCO 2) An isotope of calcium has a mass number of 44. How many neutrons are present in this isotope? 11 20 24 44 64 See Chapter 3. The atomic number of Ca is 20. 44 - 20 = 24 neutrons. [Show More]
Last updated: 2 years ago
Preview 1 out of 4 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 07, 2022
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Nov 07, 2022
Downloads
0
Views
49



 Questions and Answers 100% VERIFIED.png)
 Questions and Answers 100% correct Solutions.png)






.png)