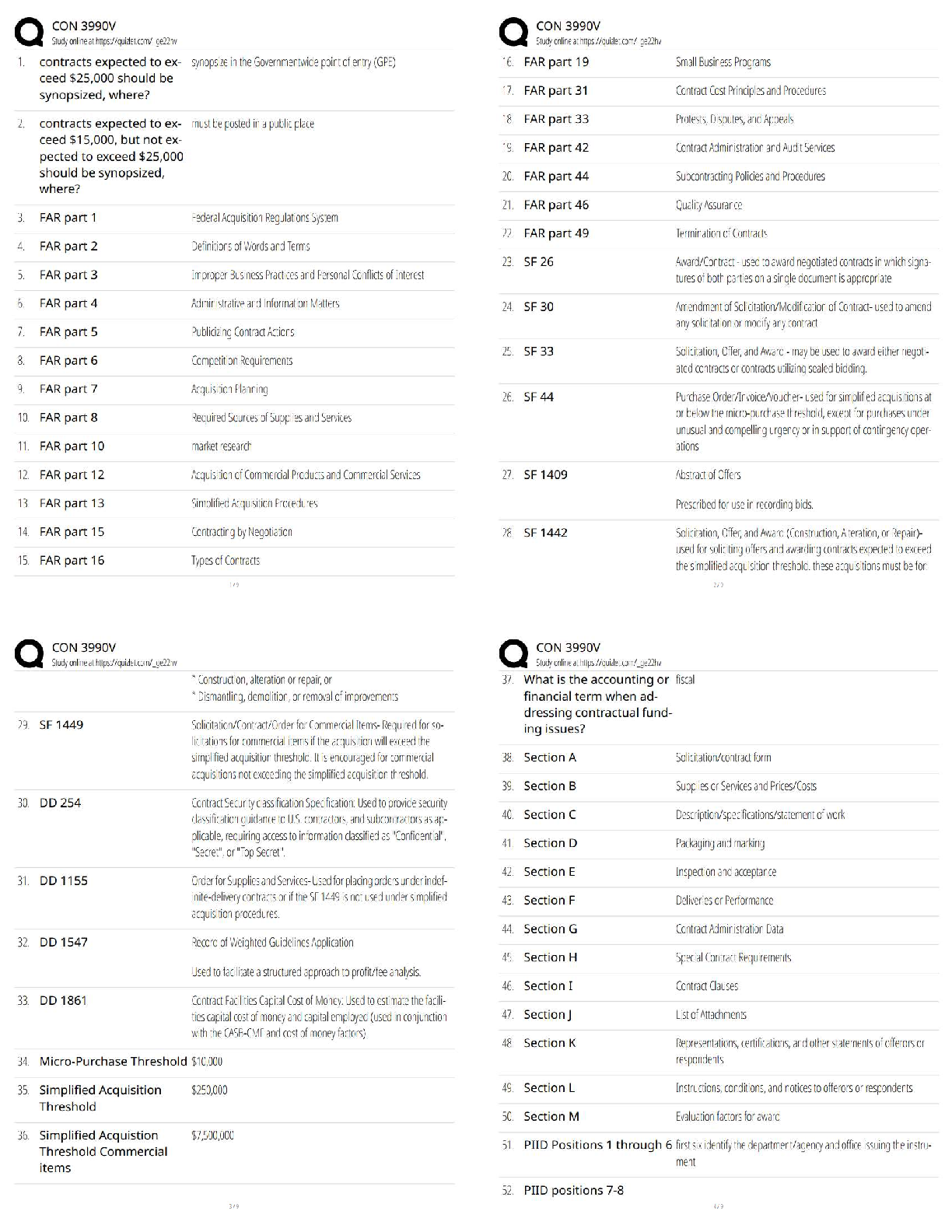
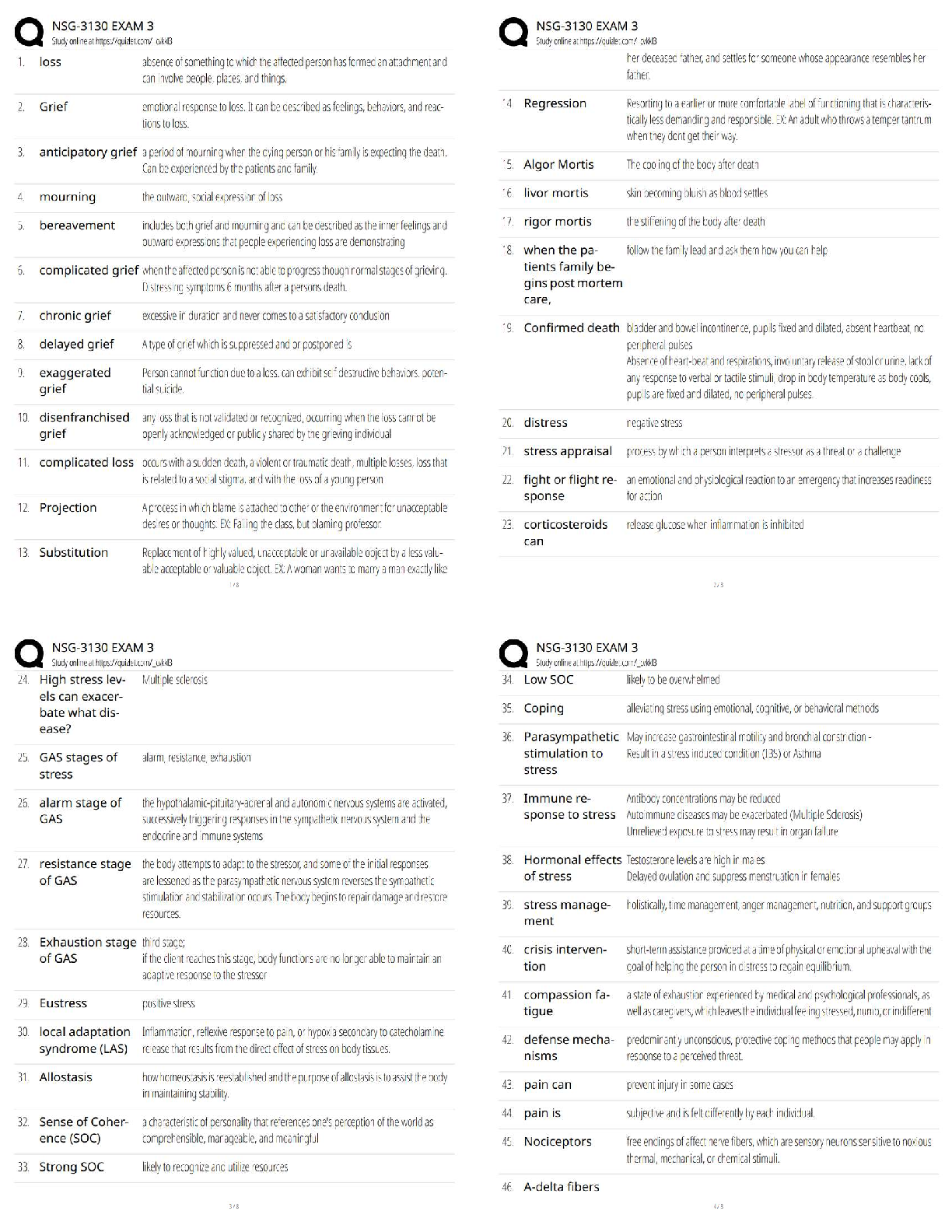
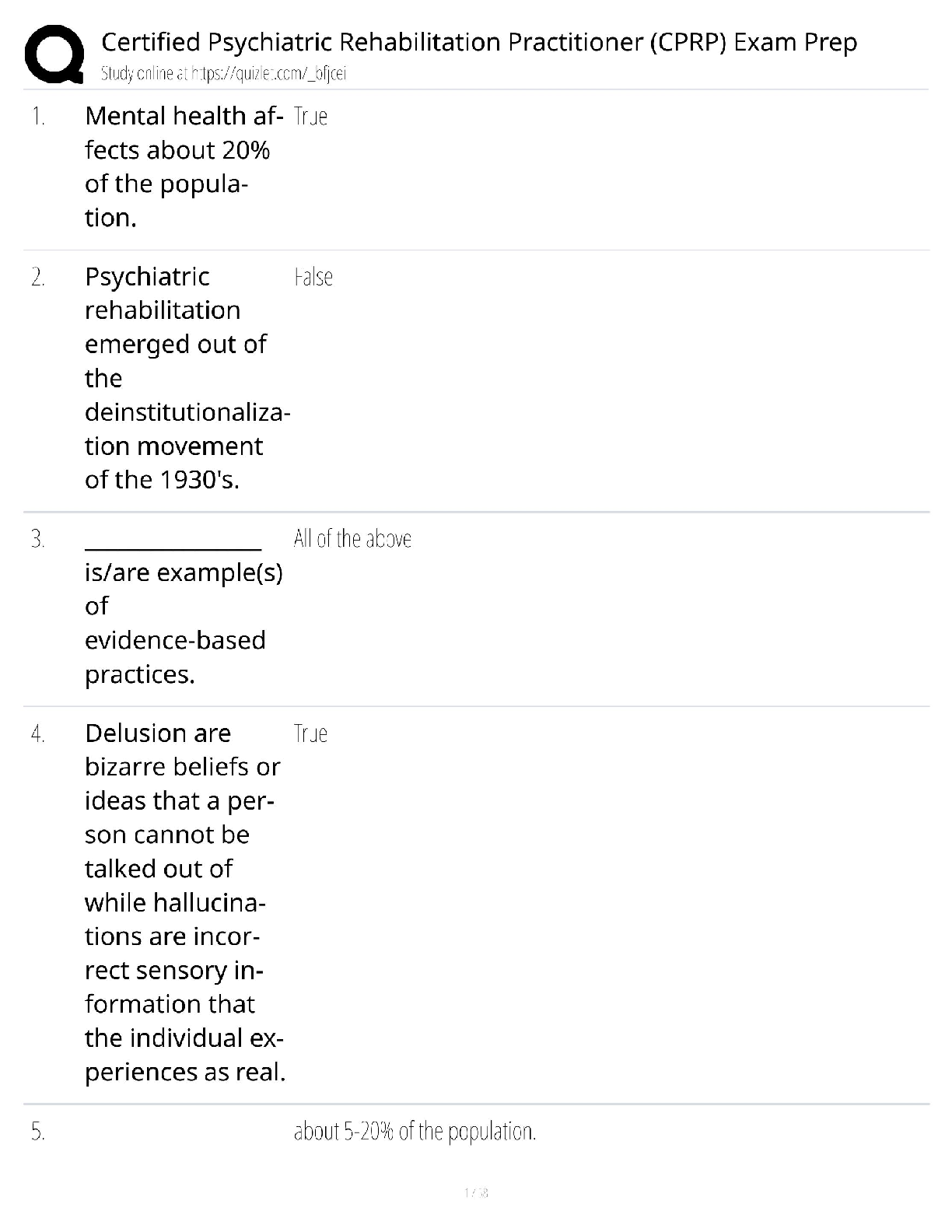
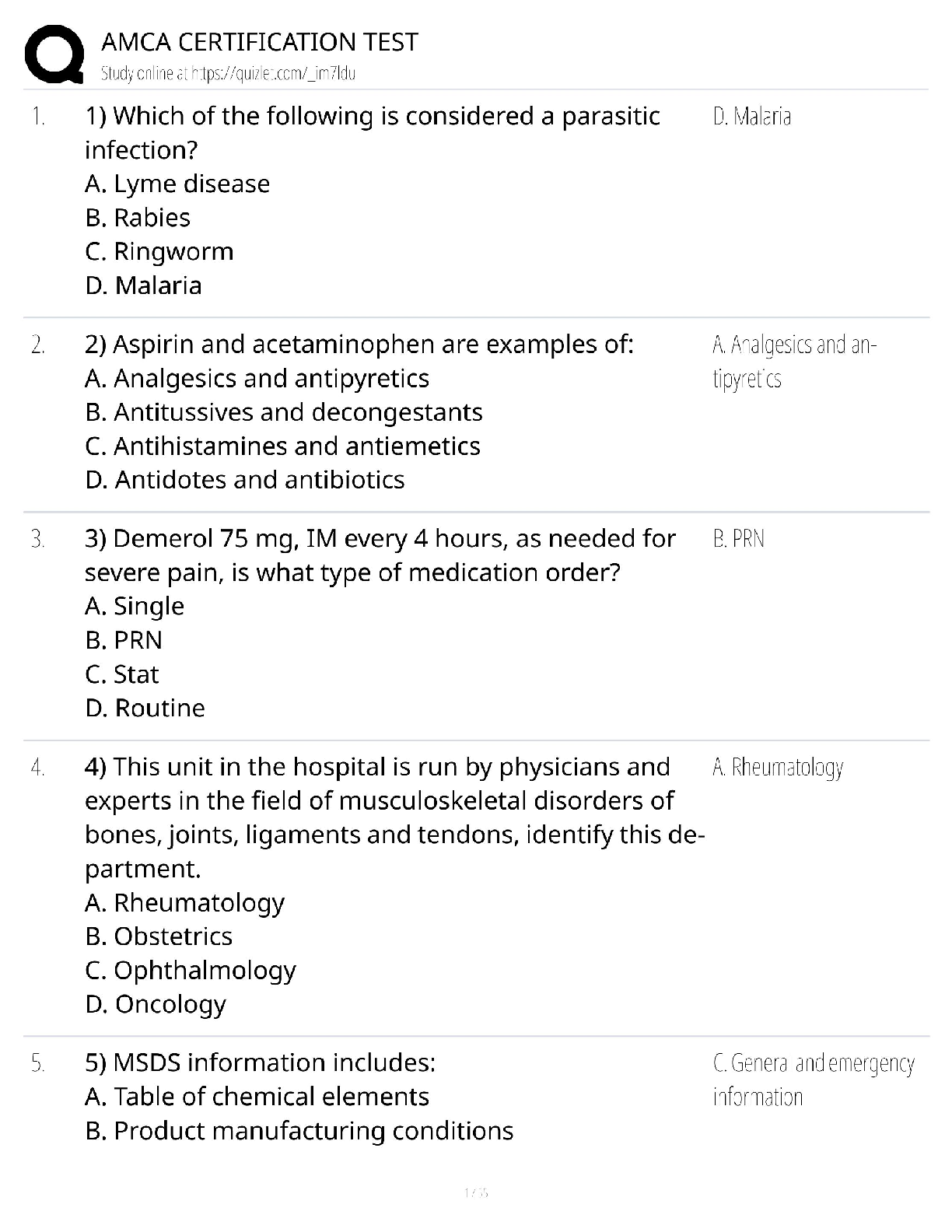
Chemistry > QUESTIONS & ANSWERS > CHEM101 Topic 6: Quantum Theory[ALL ANSWERS 100% CORRECT] (All)
CHEM101 Topic 6: Quantum Theory[ALL ANSWERS 100% CORRECT]
Document Content and Description Below
Last updated: 3 years ago
Preview 1 out of 13 pages
![Preview image of CHEM101 Topic 6: Quantum Theory[ALL ANSWERS 100% CORRECT] document](https://scholarfriends.com/storage/EXAM 2.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

CHEM101 QUESTIONS AND ANSWERS [ALREADY PASSED]
CHEM101 Topic 3: Stoichiometry[ALL ANWERS 100% CORRECT] CHEM101 Topic 3: Stoichiometry[ GRADED A+] CHEM101 Topic 6: Quantum Theory[ALL ANSWERS 100% CORRECT] CHEM101 Topic 9: Properties of Gases[ALL...
By QuizMaster82 3 years ago
$26.5
6
Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
13
Written in
All
Additional information
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
101