Chemistry > QUESTIONS & ANSWERS > CHEM101 Topic 16: Nonmetallic Elements and Organic Chemistry[GRADED A+] (All)
CHEM101 Topic 16: Nonmetallic Elements and Organic Chemistry[GRADED A+]
Document Content and Description Below
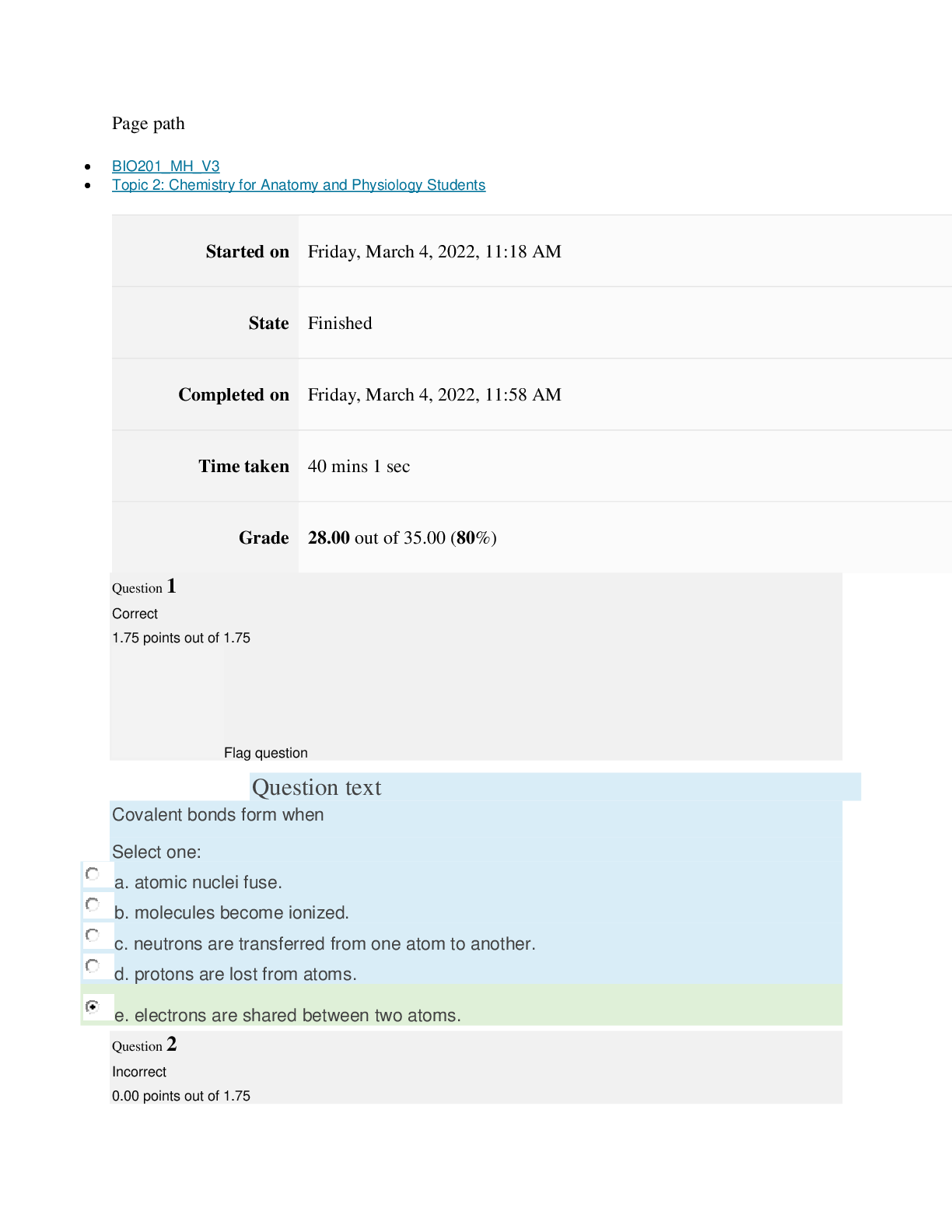
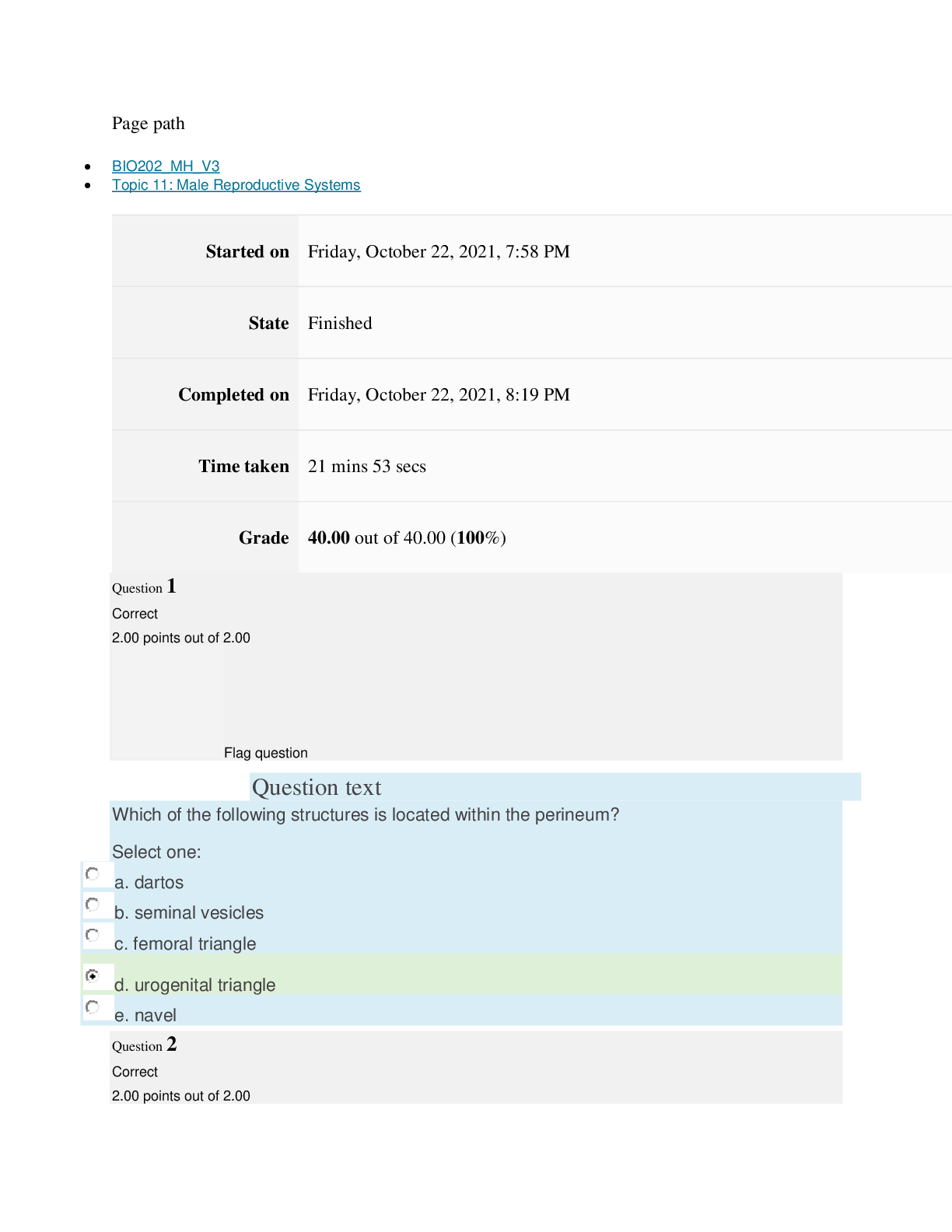
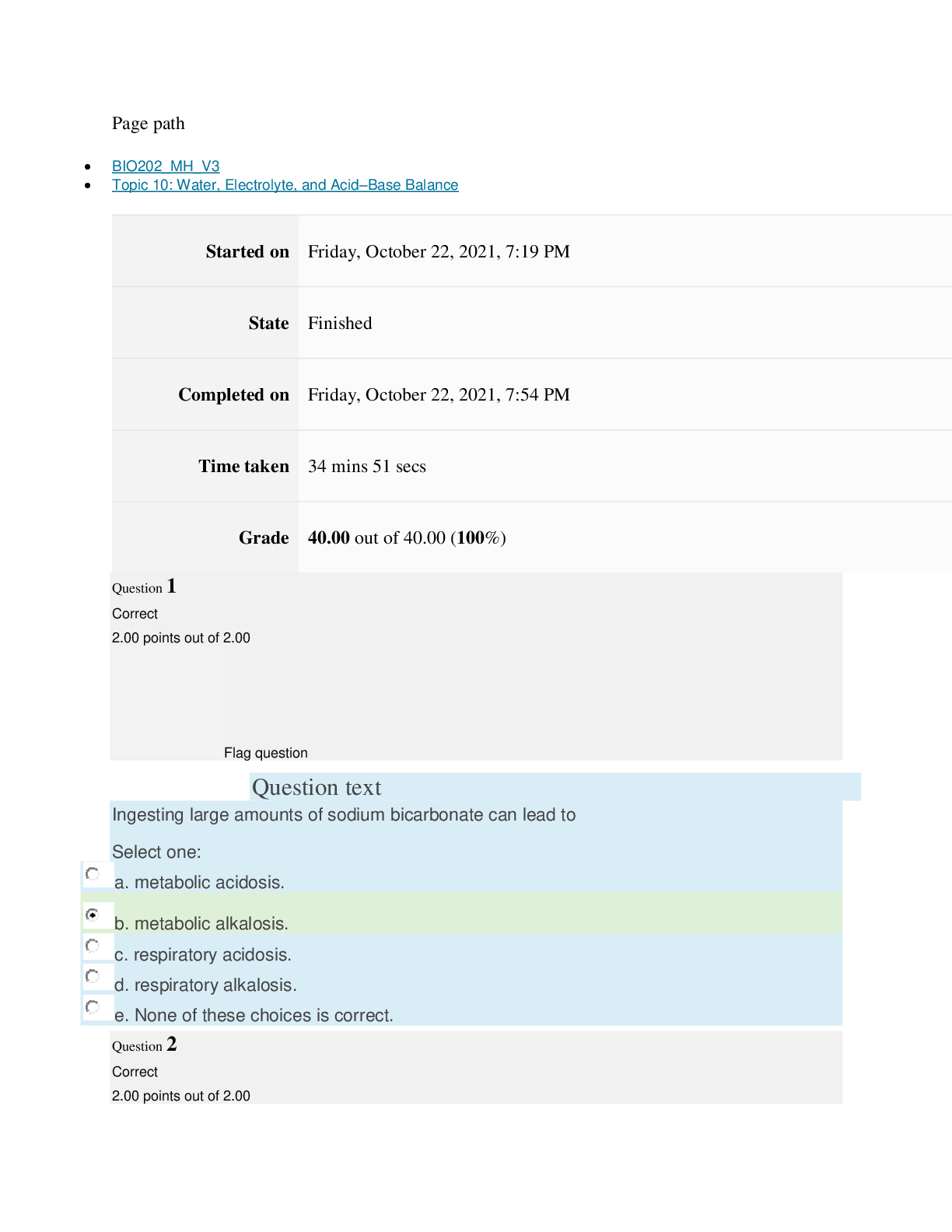
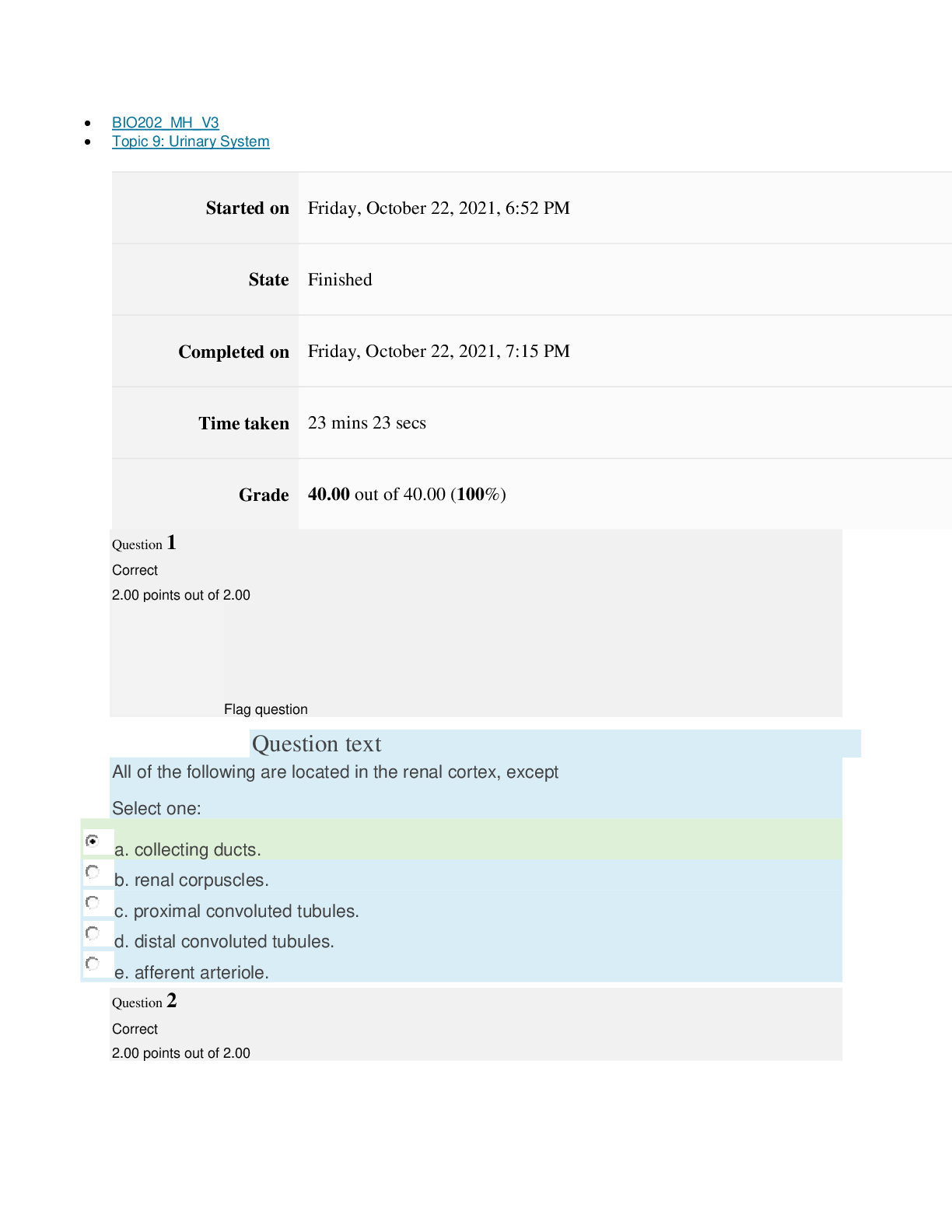
Page path CHEM101_MH_V4 Topic 16: Nonmetallic Elements and Organic Chemistry Started on Friday, April 15, 2022, 9:09 PM State Finished Completed on Friday, April 15, 2022, 9:31 PM Time take... n 22 mins 2 secs Grade 110.40 out of 115.00 (96%) Question 1 Correct 4.60 points out of 4.60 Flag question Question text What is ΔS° for the following reaction? 4Cr(s) + 3O2(g) → 2Cr2O3(s) Substance Cr(s) O2(g) Cr2O3(s) S°(J/K • mol) 23.77 205.138 80.65 Select one: Flag question Question 2 Correct 4.60 points out of 4.60 Flag question Question 3 Correct 4.60 points out of 4.60 Question text Question text Which is the thermodynamic condition for a spontaneous process at constant T and P? c. ΔG < 0 Select one: a. ΔS > 0 b. ΔS < 0 c. is always endothermic. d. does not involve any heat exchange with surroundings. b. may be exothermic or endothermic. a. is always exothermic. A spontaneous reaction Select one: b. –148.26 J/K • mol c. +148.26 J/K • mol d. +66.22 J/K • mol e. +871.80 J/K • mol a. –549.19 J/K • mol Flag question Question 4 Correct 4.60 points out of 4.60 d. 6, product side e. 8, reactant side c. 6, reactant side Select one: a. 4, reactant side b. 4, product side Question text Consider the following redox equation. Mn(OH)2(s) + MnO4 (aq) → MnO4 (aq) (basic solution) – 2– When the equation is balanced with the smallest whole number coefficients, what is the coefficient for OH–(aq) and on which side of the equation is OH–(aq) present? Question text Which statement is correct? c. Both oxidation and reduction make take place at the cathode, depending on the cell. d. The cathode is always positive. [Show More]
Last updated: 2 years ago
Preview 1 out of 14 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
14
Written in
Additional information
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
100