Chemistry > QUESTIONS & ANSWERS > CHEM101 Topic 16: Nonmetallic Elements and Organic Chemistry[GRADED A+] (All)
CHEM101 Topic 16: Nonmetallic Elements and Organic Chemistry[GRADED A+]
Document Content and Description Below
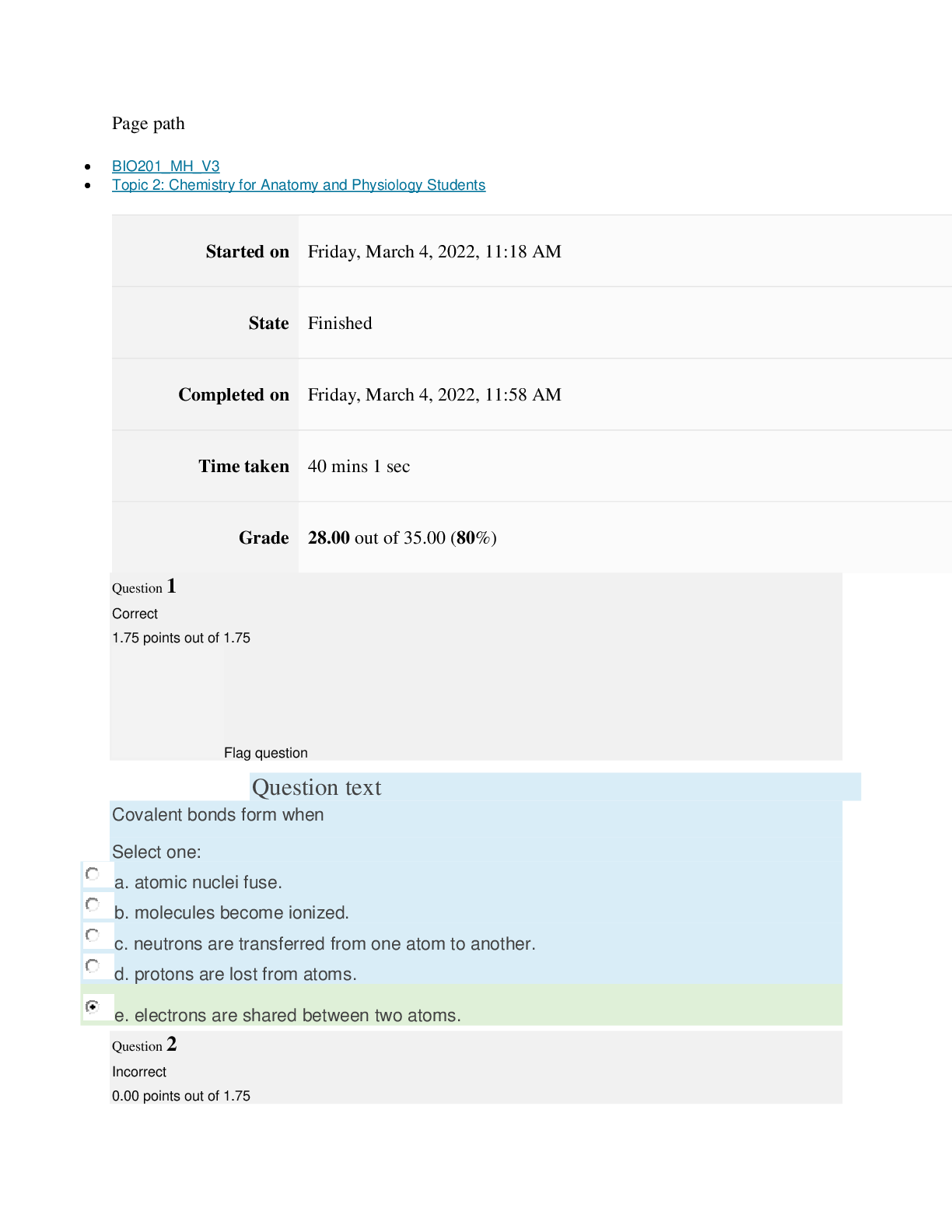
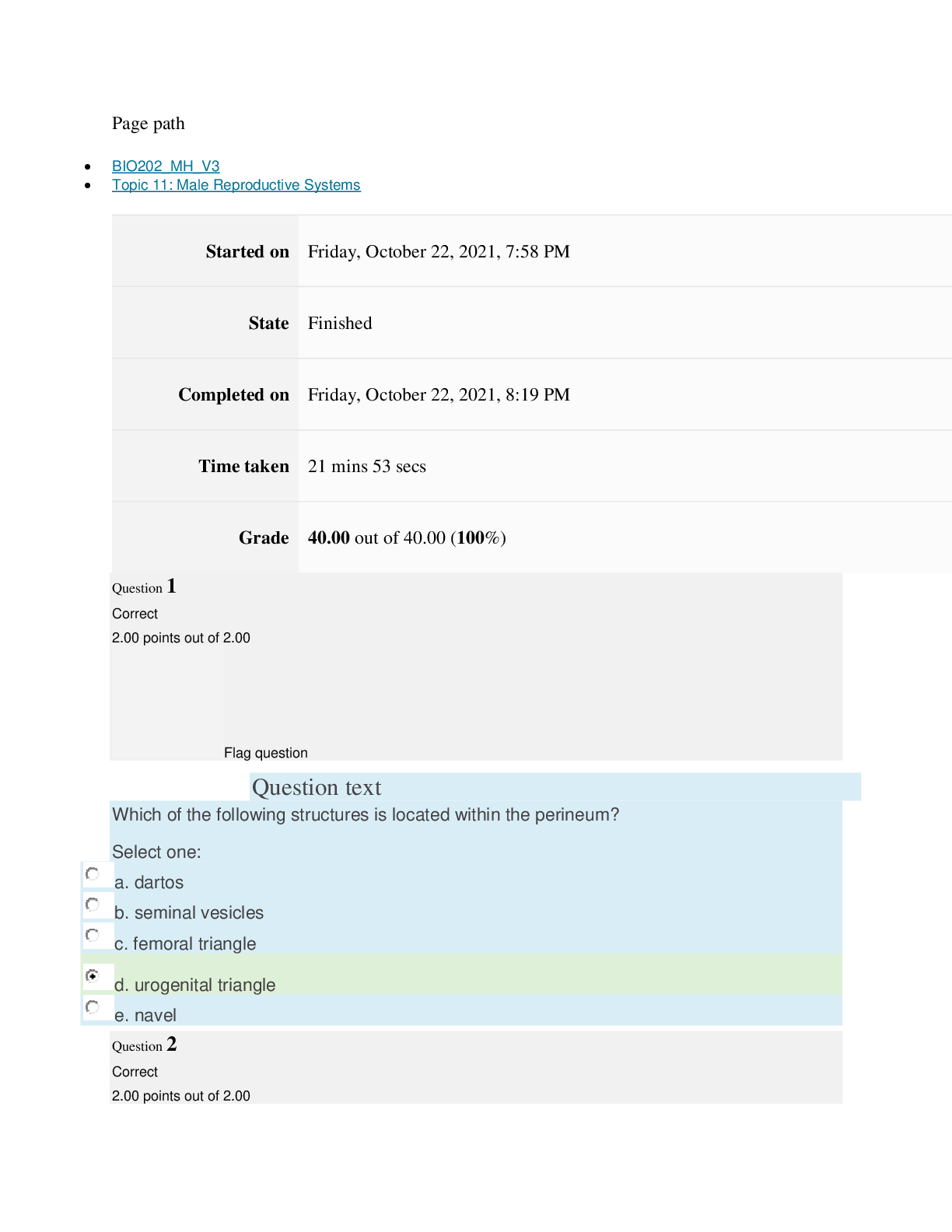
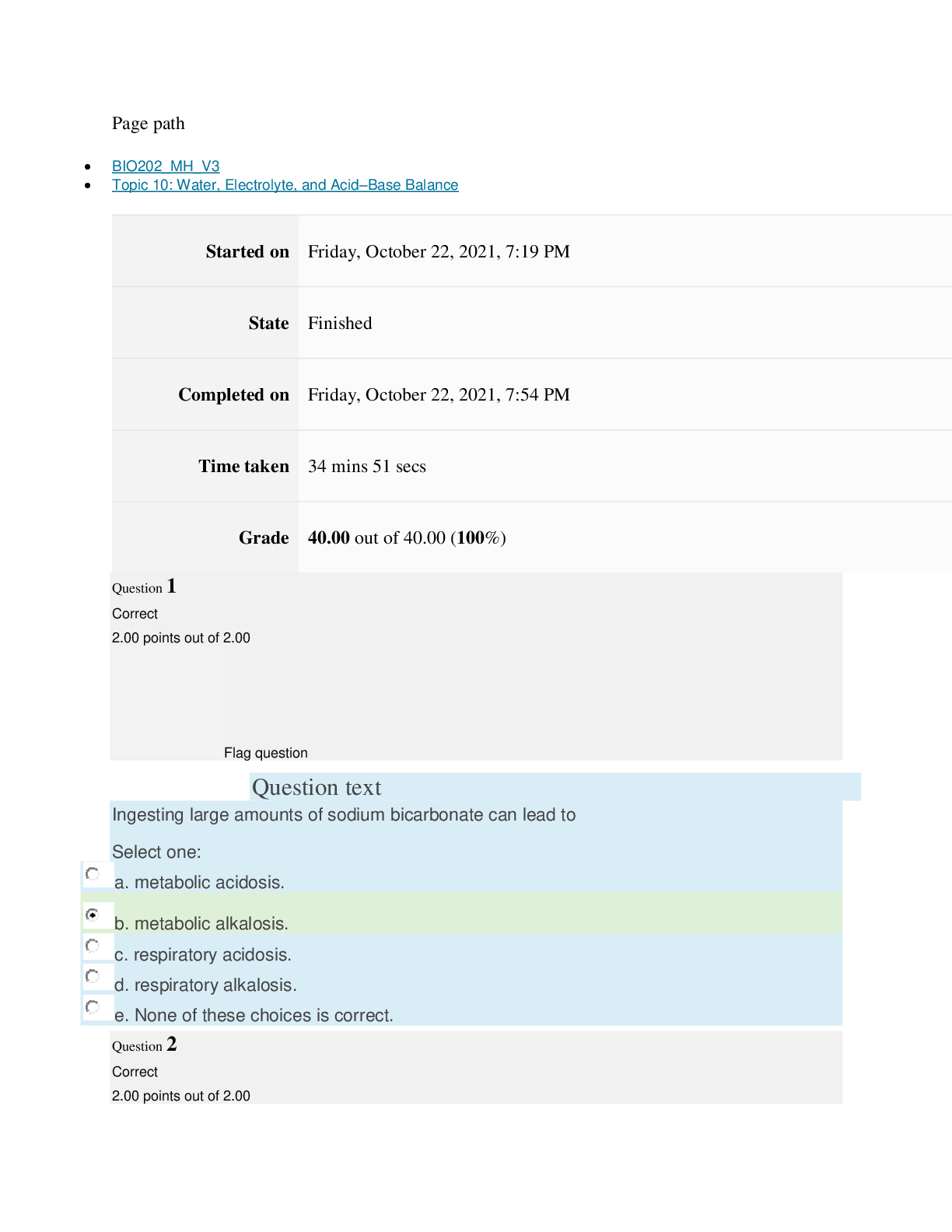
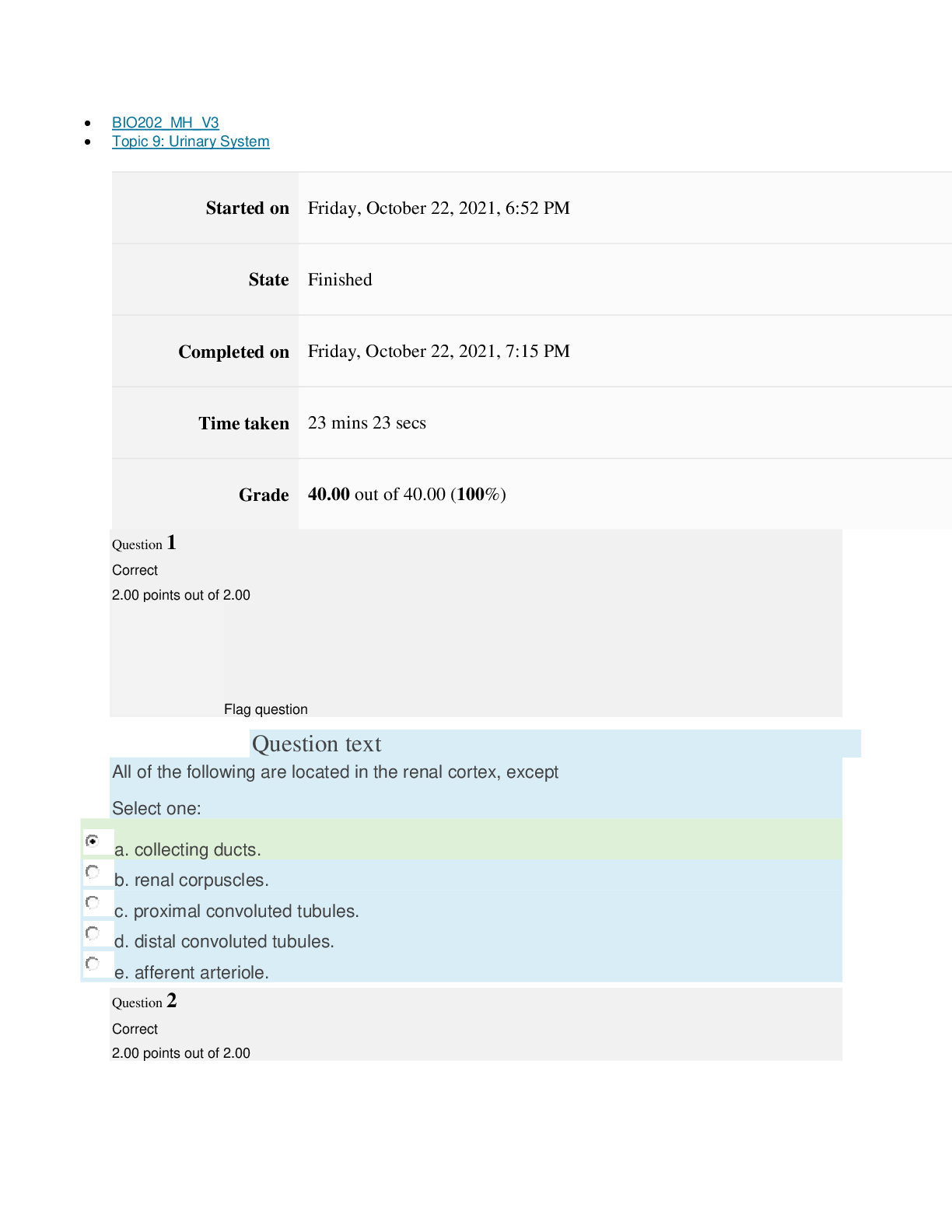
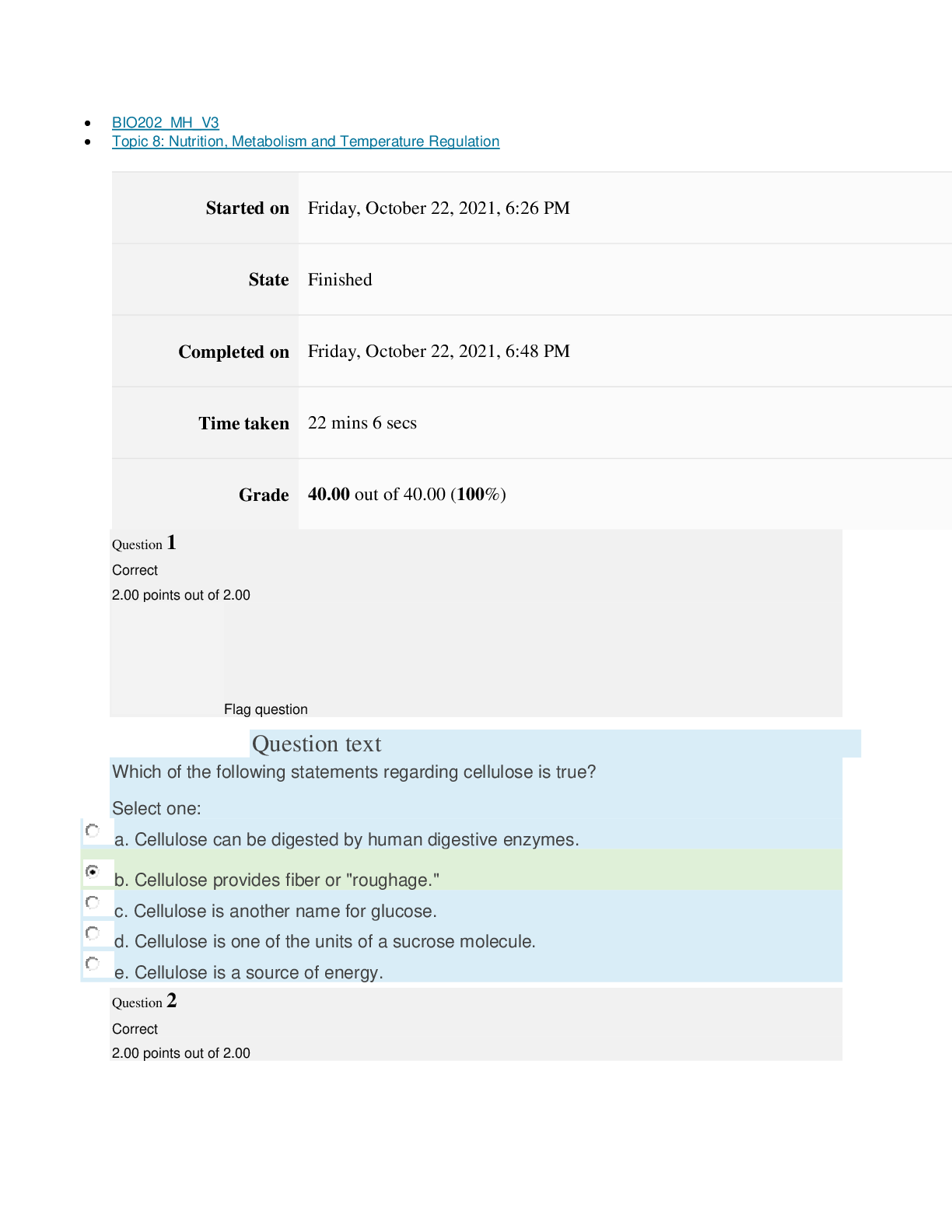
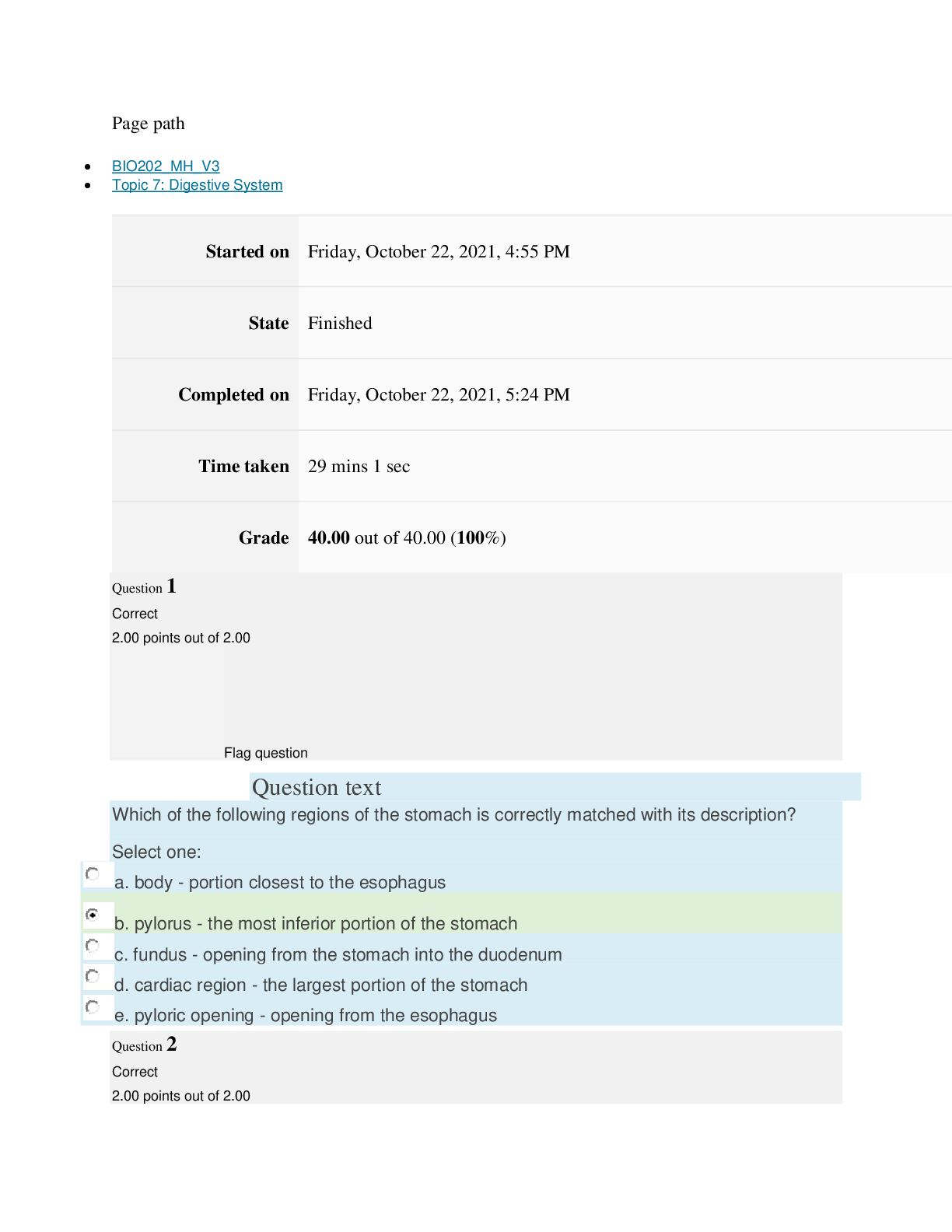
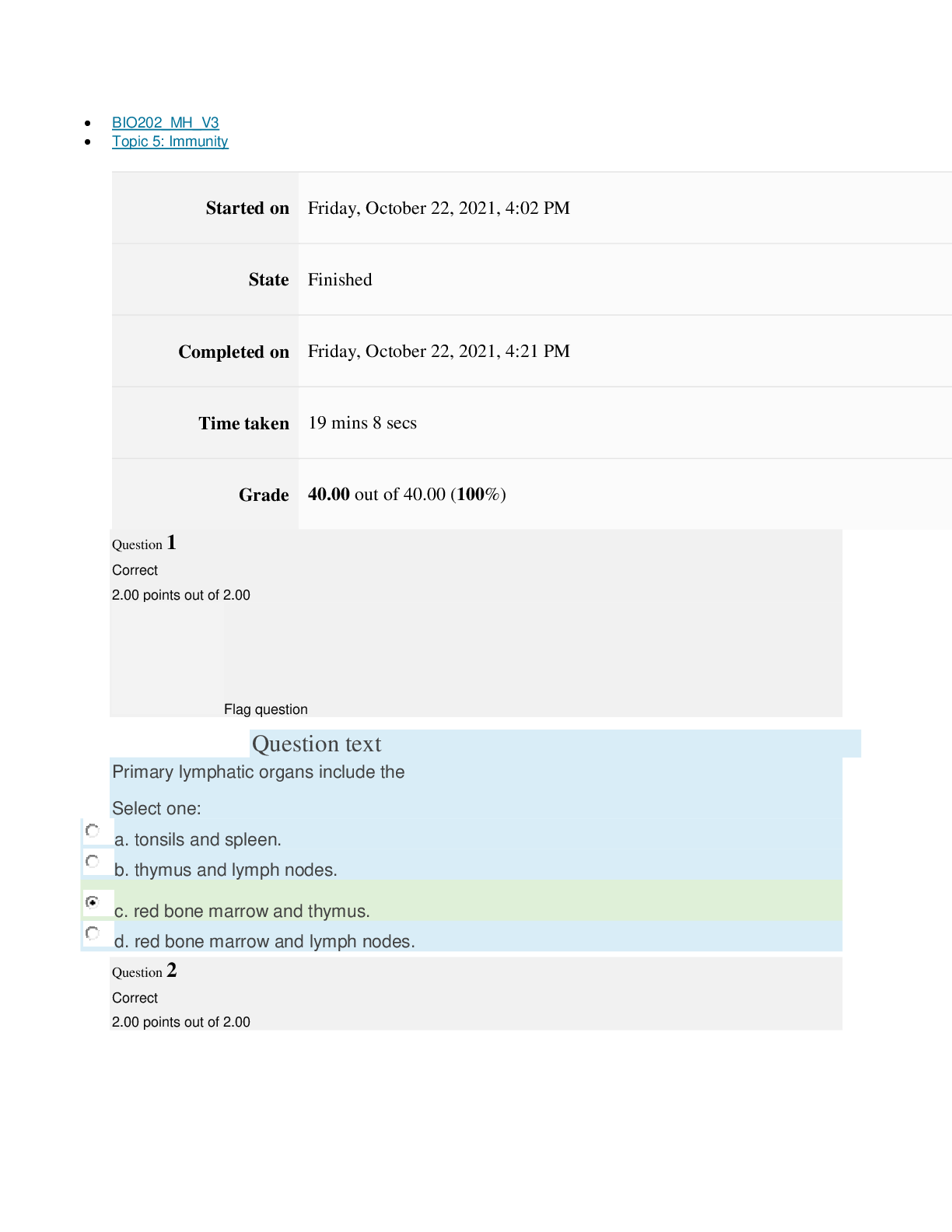
path CHEM101_MH_V4 Topic 16: Nonmetallic Elements and Organic Chemistry Started on Thursday, March 24, 2022, 11:12 AM State Finished Completed on Thursday, March 24, 2022, 11:48 AM Time tak... en 36 mins 25 secs Grade 110.40 out of 115.00 (96%) Question 1 Correct 4.60 points out of 4.60 Flag question Question text What is ΔS° at 298 K for the following reaction? Fe2O3(s) + 3CO(g) → 3CO2(g) + 2Fe(s) Substance ΔG°f(kJ/mol) ΔH°f(kJ/mol) Fe2O3(s) –741.0 –822.2 CO(g) –137.2 –110.5 CO2(g) –394.4 –393.5 Question text Question text Ozone (O3) in the atmosphere can react with nitric oxide (NO) as follows. O3(g) + NO(g) → NO2(g) + O2(g). ΔH°rxn = –199 kJ/mol, ΔS°rxn = –4.1 J/K • mol Flag question Question 3 Correct 4.60 points out of 4.60 d. system heats up but there is not change in the energy of surroundings. e. energy content of neither system nor surroundings changes. c. system loses energy while surroundings heat up. a. system heats up while surroundings lose energy. b. surroundings heat up, but there is no change in system’s energy. In a spontaneous exothermic process, Select one: Flag question Question 2 Correct 4.60 points out of 4.60 b. –12.7 J/K • mol c. 527.9 J/K • mol d. 157.2 J/K • mol e. 4.6 J/K • mol a. 12.7 J/K • mol Select one: What is ΔG°rxn for this reaction at 25°C? Select one: Question text Which is not a redox reaction? Select one: Question text What is the name given to the experimental apparatus for generating electricity through the use of a spontaneous reaction? Flag question Question 5 Correct 4.60 points out of 4.60 b. C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) c. Na6FeCl8(s) + 2Na(l) → 8NaCl(s) + Fe(s) d. 2H2O2(aq) → 2H2O(l) + O2(g) e. CO2(g) + H2(g) → CO(g) + H2O(g) 2 3+ + – 4 a. Al(OH) (aq) + 4H (aq) → Al (aq) + 4H O(l) e. –198 kJ/mol a. 1020 kJ/mol b. –1.22 × 103 kJ/mol c. 2.00 × 103 kJ/mol d. –1.42 × 103 kJ/mol Flag question Question 4 Correct 4.60 points out of 4.60 b. Galvanic cell [Show More]
Last updated: 2 years ago
Preview 1 out of 15 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 06, 2022
Number of pages
15
Written in
Additional information
This document has been written for:
Uploaded
Dec 06, 2022
Downloads
0
Views
110