Chemistry > Lab Report > LAB 6- 225 - CUNY Hunter College CHEM 225 (All)
LAB 6- 225 - CUNY Hunter College CHEM 225
Document Content and Description Below
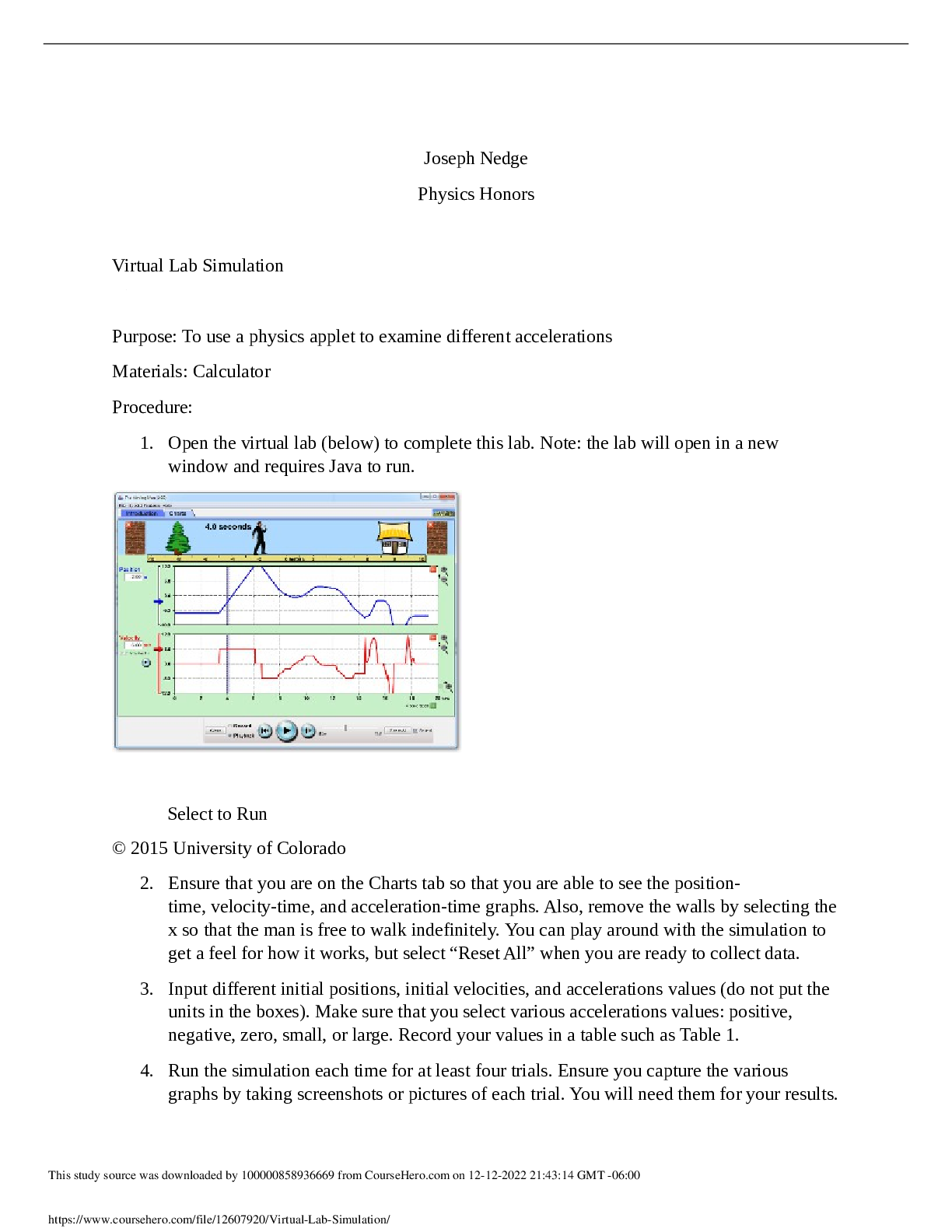
Experiment 6: Unknown Binary Mixture Patricia G. May 17, 2019 Introduction Identifying unknown compound is a very important study for chemical scientists. Chemists use many different techniques i ... n identifying unknown compounds that are used in many different sectors of studies. Unknown compounds are constantly identified in the pharmaceutical industries, forensic departments, healthcare and many more. Several tests are performed to ensure that there is a qualitative analysis in identifying the unknown compounds. In this experiment, an unknown binary mixture was separated and purified, and the compounds of the mixture were determined. The first step performed in identifying the binary compound is to do the preliminary examination of the unknown. The state, odor, and color of the mixture is observed and noted. Next, different technique is performed to separate the unknown binary mixture. Liquid-liquid mixtures are separated via acid base extraction, while solid-liquid mixtures are separated via fractional or simple distillation and crystallization. To obtain the pure solid compound of a binary mixture, the solid is crystalized by using an ideal solvent that gives the borderline solubility of the solid. In crystallization of the solid, the solid compound of the binary mixture dissolves in the ideal hot solvent and precipitates in ice bath. The pure solid is finally collected by vacuum filtration and the melting point of the pure solid compound is obtained. The boiling point of pure liquid was obtained by performing fractional distillation. The boiling temperature of a pure liquid compound in distillation can be obtained when its vapor pressure is equal to the vapor pressure of the atmosphere. For a pure liquid the boiling temperature should remain constant for the duration of the distillation. Nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy are the major spectroscopic techniques used by organic chemists to identify functional groups in molecules and to determine the structure of a compound. It is essential to analyze qualitative tests for organic functional groups to gain insight of the functional groups present in the unknown compounds. A series of solubility tests were also conducted to detect the presence of an acid, base or neutral substance in the pure compounds. Lastly, a derivative is formed for each unknown to verify that the correct identity is determined. A derivative is a new compound that forms from the unknown however, with a new functional group. The similarity between the melting point of the purified derivative to the literature melting point of the derivative proves successful identification of the unknown compound of the binary mixture. Observation and Results Part A: Preliminary Examination Unknown 94 is a liquid-solid binary mixture with a very strong odor. The solid is white in color and the liquid is clear in color. The compound has low volatility; it does not vaporize at room temperature. Part B: Separation & Purification Simple distillation was used to separate the pure liquid from impure liquid. While, the pure solid was obtained by crystalizing the impure solid. To find the pure liquid, a simple distillation apparatus was set up with the unknown liquid in distilling flask. The first drop of distillate was obtained at 81 , and then most of the pure liquid was obtained at 113 where the temperature ℃ ℃ reached its maximum point indicating the boiling point of the unknown liquid. The last few drops were collected in a separate beaker when the temperature started to decrease. The leftover liquid in distilling flask when cooled to room temperature became solid precipitate, which suggested that all impurities were separated from unknown liquid. The unknown solid was crystalized with the correct solvent to obtain the pure solid. In order to determine the correct solvent for recrystallizing, multiple solvents were tested, however only hot methanol completely dissolved the unknown solid. After determining the correct solvent for the unknown, all the solid were dissolved in hot methanol and precipitated in ice bath. The final product of the pure solid was obtained by vacuum filtrating the precipitate. The pure solid had a crystal-like appearance with a strong odor. The melting point od the pure solid is 36-39 . ℃ - Boiling point of pure Liquid: 113℃ - Melting point of pure solid: 36-39 . ℃ Pact C: Solubility Tests Solubility Test for Unknown S [Show More]
Last updated: 3 years ago
Preview 1 out of 15 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 13, 2022
Number of pages
15
Written in
All
Additional information
This document has been written for:
Uploaded
Dec 13, 2022
Downloads
0
Views
126













.png)





.png)



