Chemistry > QUESTIONS & ANSWERS > Weekly Notes > CHEM 177L 2017_10_13_Notebook_7822 (All)
Weekly Notes > CHEM 177L 2017_10_13_Notebook_7822
Document Content and Description Below
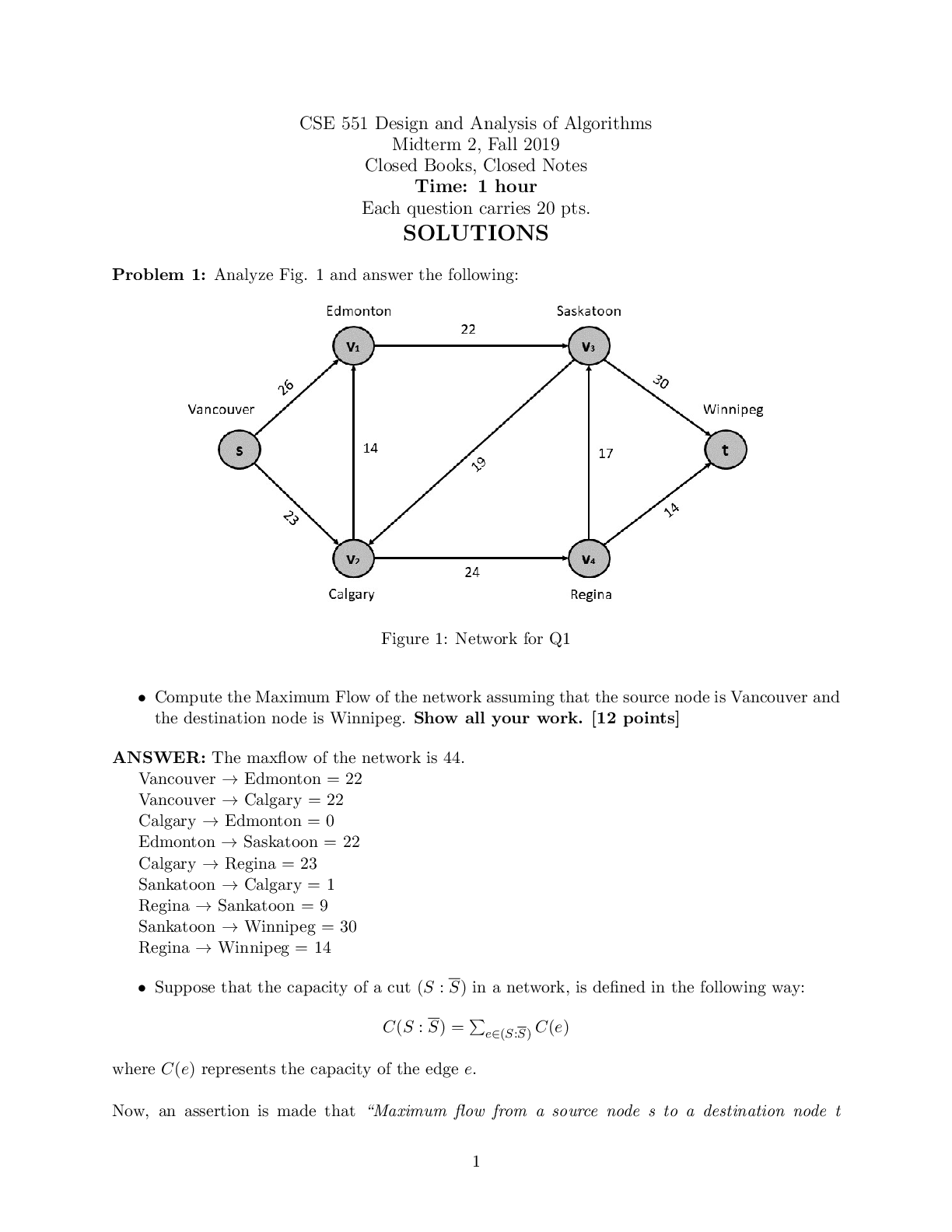
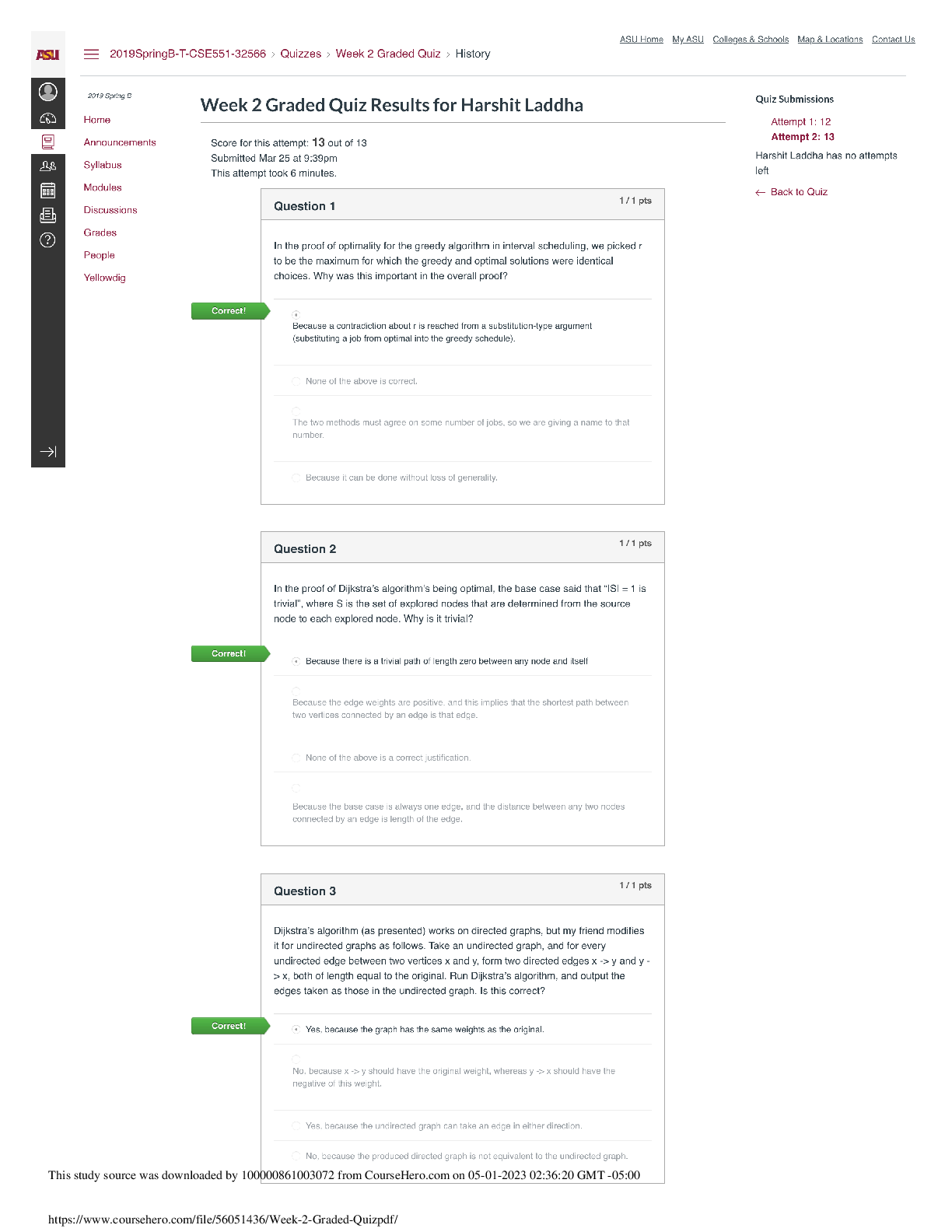
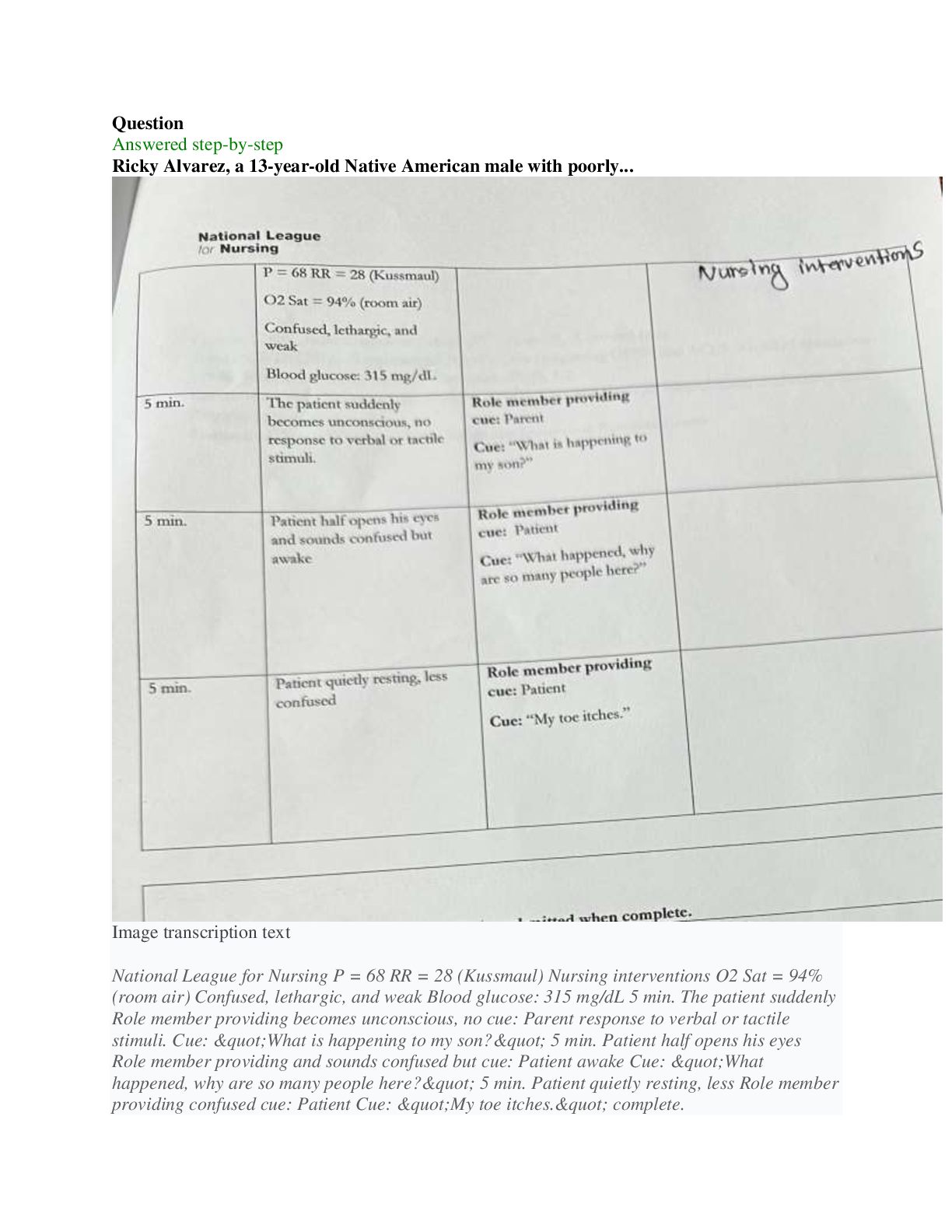
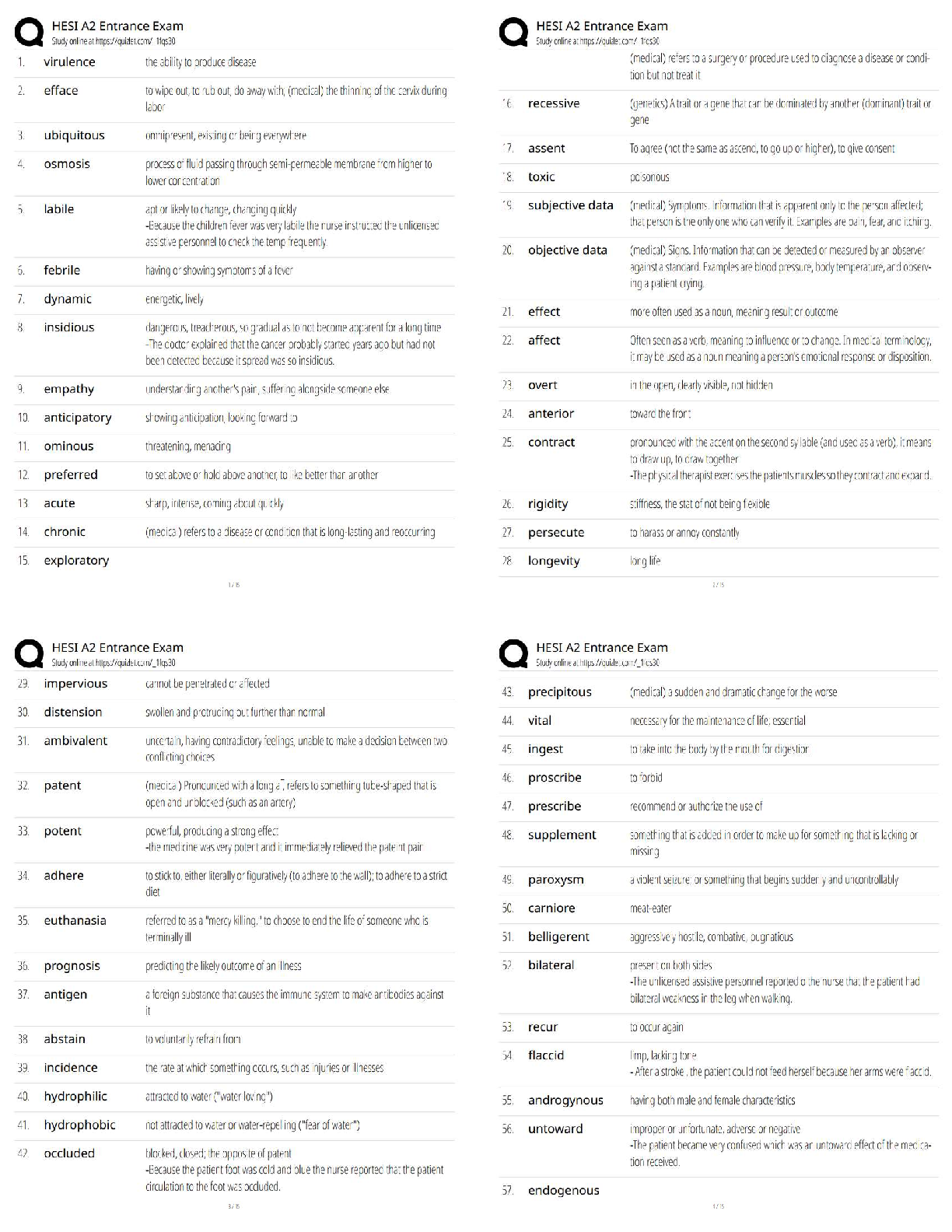
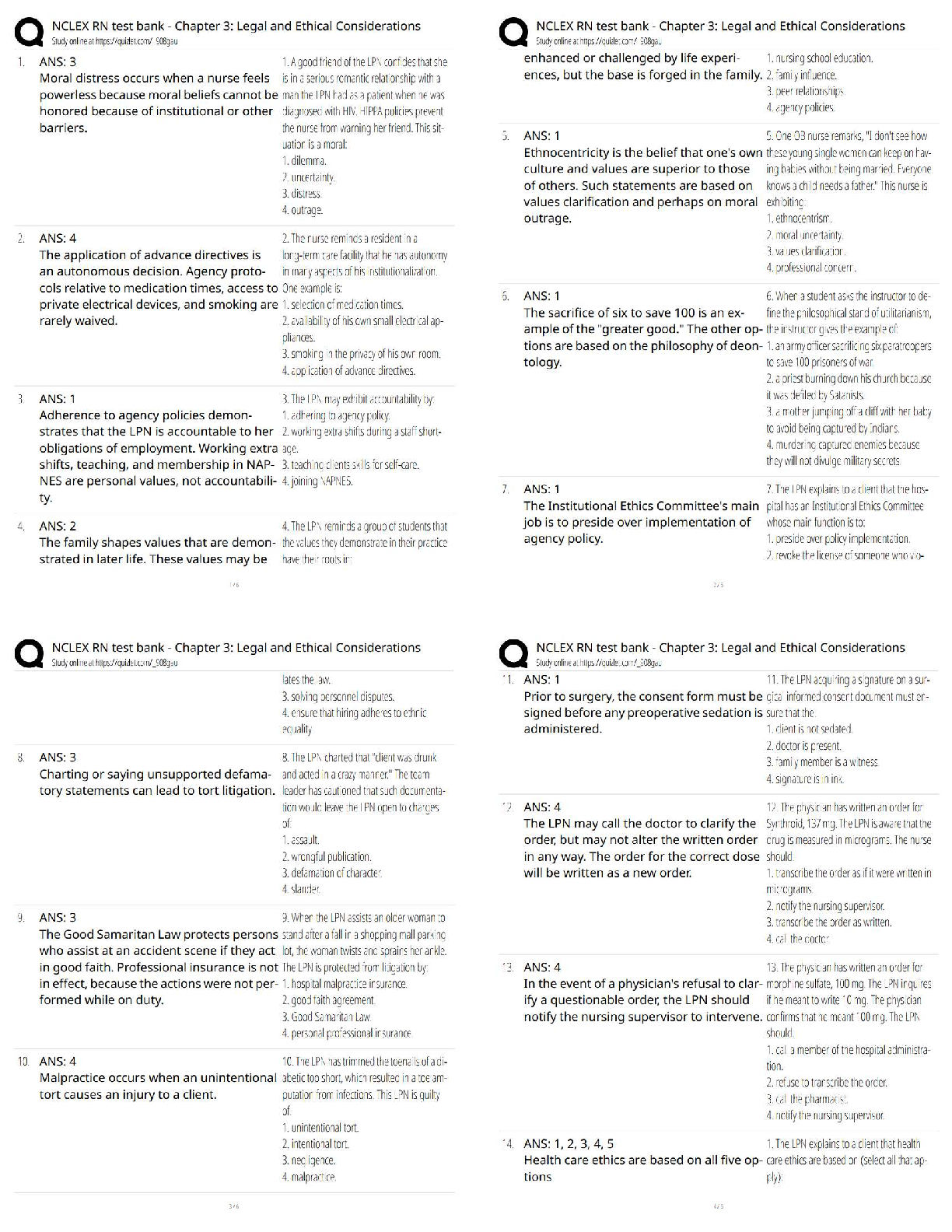
CHEM 177L 2017_10_13_notebook_7822 12 CHEM 177L Spring 2017 - Angie Burke Weekly Notes/Week 8 PDF Version generated by Angie Burke on Oct 12, 2017 @09:21 PM CDT Table of Conte ... nts Table of Contents Week 8 Week 8 Week 8 - Heat Exchange in Chemical Process Angie Burke, Lane Vaselaar, Emma Sass, Emma Arenholtz Prelab Writing Investigation Questions: 1. What kind of reactions procedure (exothermic)/ absorbs (endothermic) heat? 2. What is the sign of enthalpy for exothermic/ endothermic reactions? 3. What does the Hess's Law state? 4. Why is the Hess's Law useful? General Experimental Procedure for Part 1-3: 1. Use a solution calorimeter for making all of your measurements. Do not allow the total volume of the solution being studied to excess 80% of the total volume of the calorimeter. 2. Suspend the thermometer from a clamp attached to the ring stand. Place the calorimeter on top of the magnetic stirrer and place the stir bar in the calorimeter to keep the solution well stirred. 3. Add the first reagent to the calorimeter. Then screw the cap onto the bottle. 4. Start the stirrer at a moderate speed. Observe and record the temperature at 30-seconds intervals for several minutes to establish the initial temperature of the first reagent. Make sure not to turn on heat to avoid interfering with the reaction. 5. Note the time, then loosen the screw cap and quickly and carefully add the pre-weight sample of interest, making certain the entire sample drops directly into the first reagent. 6. Screw the cap on again and begin to record the temperature reading 30 seconds after the reactants were mixed. 7. Temperature readings continue at 30-second intervals until 10 minutes after the maximum temperature was reached. Use the temperature value that holds steady for five minutes. 8. Use Google sheets to plot graphs of your "temperature versus time" data for each reaction. 9. Gather materials: 1. Calorimeter 2. Thermometer (calibrated as outlined above) 3. Graduated cylinder 4. Hot plate 5. Magnetic stir bar 6. Automatic stirrer 7. Ice Part 1 Procedure: Accounting for other heat transfer (optional, check with TA) 1. You need at least 150 mL for thermometer to reach the water in the calorimeter and maximum volume of the calorimeter is about 400 mL. 2. Pour the water down into the drain. 3. Equations for finding the amount of heat transferred 1. q = mCΔT, where ΔT = T final - Tinitial 2. qgained = - qlost 3. q gained by cold water + q calorimeter = -q lost by warm water 1. q gained = [mCΔT]cold water 2. q calorimeter = KΔT calorimeter 3. q lost = [mCΔT] hot water 4. After substitutions: [mCΔT] cold water + kΔT calorimeter = -[mCΔT] hot water 5. Therefore: K = (-[mCΔT] hot water - [mCΔT] cool water) /ΔT calorimeter Part 2 Procedure: An acid-base reaction 1. You need at least 200 mL for thermometer to reach the solution in the calorimeter and the maximum volume of the calorimeter is about 400 mL. 2. Pour the waste into proper waste containers. 3. Equations for finding the heat of reaction from temperature change data. Week 8/ 2 of 8 1. q rxn + q solution + q calorimeter = 0 2. q calorimeter = C x ΔT = 0 3. q rxn + q solution = 0 4. ΔH rxn = qrxn / moles of limiting reagent reacted Part 3 Procedure: Dissolving salts 1. Use a mass range of 5.0 g to 8.0 g of the salts and a volume of about 250 mL 2. Dispose the waste into proper waste containers Safety Precautions: 1. Sodium hydroxide NaOH (aq) - May cause eye and skin irritation. Inhalation will produce respiratory tract irritation. Wear PPE.Use proper ventilation during reaction 2. Hydrochloric acid Hal (aq) - Corrosive. Causes eye, skin, respiratory and digestive tract burns Wear PPE. Use proper ventilation 3. Cooper (II) sulfate pentahydrate CuSO 4*5H2O(s) - May cause eye and skin irritation. Inhalation may cause respiratory tract irritation. Avoid skin and eye contact. Wash thoroughly after handling 4. Cooper (II) sulfate, anhydrous CuSO 4(s) - Hazardous in case of skin contact, eye contact, ingestion and inhalation. Avoid skin and eye contact. Wash throughly after handling. Procedure Part 2 - An acid and base solution Sodium hydroxide: 100 ml. Hydrochloric Acid: 100 ml Ti = 20.9 C in 2 mins 50 secs. Tf = 31.5 C in additional 1 mins 10 secs After the two solutions are mixed together, after 7 mins: 31.3 C; 7mins 30 secs: 31.2 C; 10 mins 31.2 C Procedure Part 3 - Dissolving salts 1. Cooper (II) sulfate pentahydrate: 5.870 g Water: T i = 21.1 C after 1 min 57 secs Solution1: T f = 21.0 C (21.0 C after 30 secs, 21.0 C after 1 mins, 21.0 C at 1 mins 30 secs. After 5 mins, the temperature stayed at 21.0 C.) 2. Cooper (II) sulfate anhydrous: 5.256 g Water: T i = 21.6 C after 3 mins 21.6 C Solution2: T f = 23.7 C ( 24.2 C after 30 secs, 24.0 C after 2 mins, 23.9 C after 2.5 mins, 23.8 C after 3 mins, 23.7 C after 7.5 mins, the temperature stayed at 23.7 C after 10 mins) Analysis Questions Week 8/ 3 of 8 Part 2 result Part 3 result Image 1 Week 8/ 4 of 8 Picture 1 - Calculations Analysis Questions Part 1 1. Calculate the calorimeter constant for the calorimeter you are using. If this part was discussed only, explain what the calorimeter constant is and what assumption you made. 1. The calorimeter constant is assumed to be zero, because it's too small to make an effect. 2. Do you think the calorimeter constant in this experiment will affect your results for the later investigations? Why or why not? 1. No. Because it's too small to cause an effect. Analysis Questions Part 2 1. Write a balanced equation for the reaction of sodium hydroxide with hydrochloric acid. Calculate the number of moles of each reagent you used in the reaction. Use the balanced equation to determine which reagent was limiting, and how many moles of it reacted (show all your calculations for full credit). Was the acid or the base the limiting reagent? . ( calculations are shown in the picture 1) 2. Calculate the value of the enthalpy change (ΔHrxn) in units of kJ/mol for the reaction of NaOH(aq) + HCl(aq). Is the reaction an endothermic or exothermic process? 1. Exothermic. ( calculations are shown in the picture 1) Analysis Questions Part 3 Discuss with your team (show your group data table) and the whole class (show class data table) and enter the answers of the following questions in your ELN. 1. Calculate the value of the enthalpy change (ΔHdiss) in units of kJ/mol for dissolving both forms of the salt (show all your calculations for full credit). Is dissolving an endothermic or exothermic 1. ( calculations are shown in the picture 1) 2. Write balanced reactions for both dissolving processes. ( calculations are shown in the picture 1) a) How do the ΔHdiss values compare for the two forms of the salt? 1. One is enothermic with a small value, Another one is exothermic with a much higher value. b) Does the difference in ΔHdiss values tell you anything about how the two salts compare? For example, which is the more stable form of the salt? 1. The one that's enthermoic is more stable, because it's a lower-energy compound that has Week 8/ 6 of 8 Angie Burke Sep 30, 2017 @03:10 PM CDT a smaller value of ΔHdiss. c) Is there a way to determine how much heat you would need to add to get from one salt to the other? 1. Heat can be determined by subtracting the ΔHdiss from both reactions. 3. How does having waters of hydration affect the enthalpy for dissolving? 1. Water changes how stable the solution is. Reflective Writing Week 8/ 7 of 8 Angie Burke Oct 12, 2017 @08:43 PM CDT Explain: Investigation Questions: 1. What kind of reactions procedure (exothermic)/ absorbs (endothermic) heat? 1. Endothermic reaction absorbs heat. Exothermic reaction produces heat. 2. What is the sign of enthalpy for exothermic/ endothermic reactions? 1. exothermic - negative; endothermic - positive 3. What does the Hess's Law state? 1. No matter how many stages of steps of a reaction takes, the total enthalpy change is the sum of all changes. 4. Why is the Hess's Law useful? 1. It allows the enthalpy to be calculated even if it can't be measured directly. Post Discussion Questions q cal is approximately = 0: Why is this a good assumption? Because it's not big enough to make any effect Which q did you actually measure? qrxn or qsolution? q reaction Can you measure the other q? Why or why not? If yes, how? No. Because only calculation can be done on q solution. Why is the qrxn= - qsolution? Define what the system and the surroundings are for each experiment. You can neither create or destory the energy. The water is the surrounding. The NaOH and HCl are the system How do volume and mass influence ΔH e q? It affects how much compacity you have to measure the heat. How did you decide which was the limiting reagent in each experiment? Compare the moles of the reagents. How did you applied the Hess’s Law? We measured how much heat the system lost/ absorbed with different types of solutions, and how much heat that's been absorbed/ lost by surrounding. What type of errors might affect your results? (Please avoid mentioning “Human error” and try to be as specific as possible) There are water leftover, spills, an assumption we made on q (states that it doesn't matter). Evaluate: 1. Do your answers to investigation questions make sense? Are they consistent with what is already known about chemistry? How do your conclusions tie in with what you are learning in lecture? Yes. They are consistent with what we have been learning. From lectures, we learned that endothermic reactions are positive and exothermic reactions are negative. 2. Are your results consistent with your entire class? If not, why not? What would you do differently in order to get even better results? Saying anything that has to do with human error will not receive any points. They are not all consistent with class data. The reasons might due to water leftover, spills and inaccurate measurements that caused the errors to happen. Extend: 1. How does what you have learned in lab today tie in with anything outside your chemistry lab and lecture? Exothermic and endothermic happens everywhere in normal life, such as cooking, light a candle, and freeze something in the refrigerator. Week 8/ 8 of 8 [Show More]
Last updated: 2 years ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$5.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 06, 2023
Number of pages
8
Written in
All
Additional information
This document has been written for:
Uploaded
Jan 06, 2023
Downloads
0
Views
82