BioChemistry > EXAM > Biochemistry C 785 Readiness Check 2020 – Western Governors University | Biochemistry C785 Readine (All)
Biochemistry C 785 Readiness Check 2020 – Western Governors University | Biochemistry C785 Readiness Check {A Grade}
Document Content and Description Below
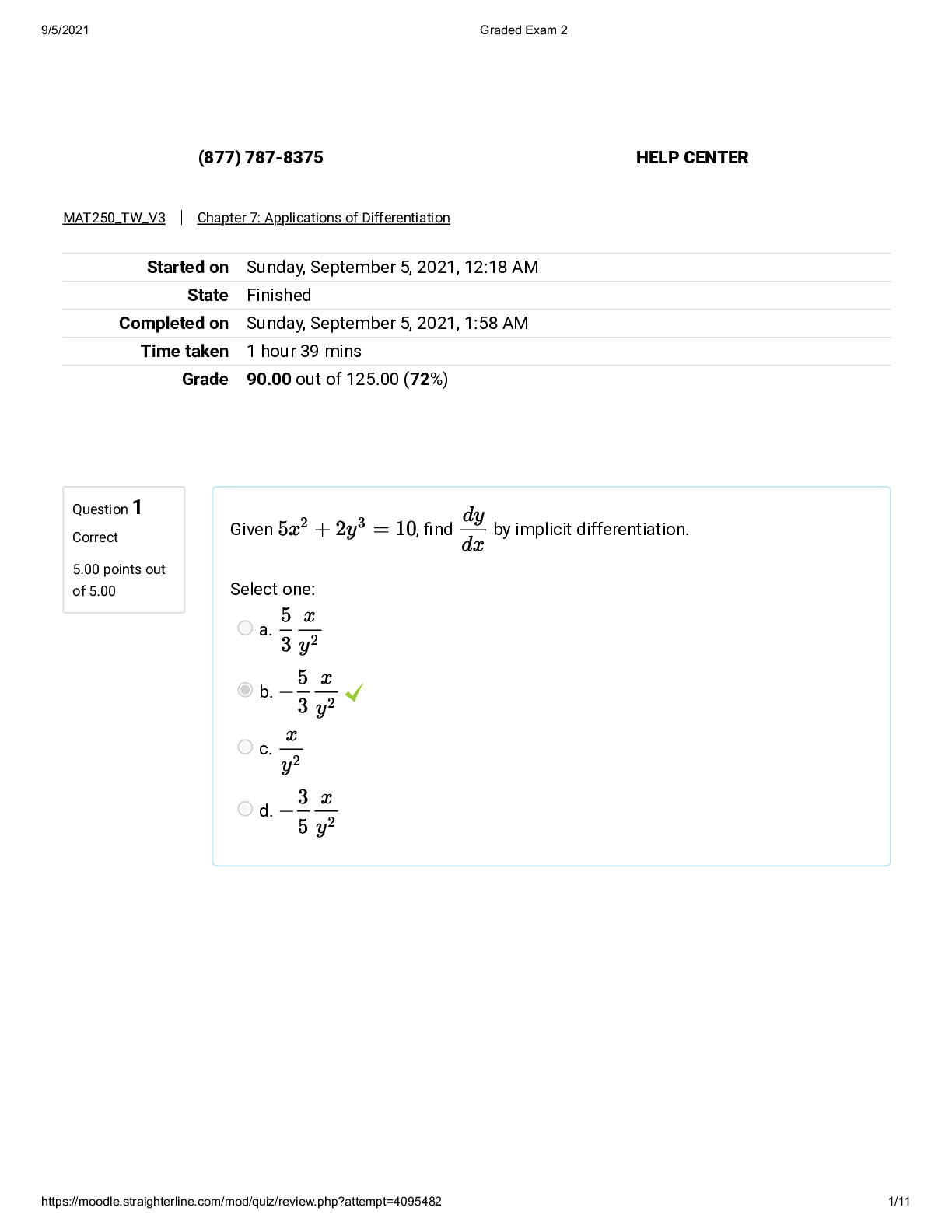
Choose the correct coding strand (non-template) from this template (non-coding) DNA: 5' ACG GTA 3' (1/1 Points) 5' UAC CGU 3' 3' UAC CGU 5' 3' TAC CGT 5' 5' TAC CGT 3' Correct! First, note t ... hat we are asked for a template/non-coding strand of DNA. DNA uses the base T where RNA uses the base U. Therefore, we can eliminate the two answers containing U because they must be RNA and we want DNA. Then, recall that any strands of nucleic acid that bind to each other (base pairing; A-T/U or C-G) must be each be running in opposite directions (antiparallel; a strand running 5'-->3 is bound to a strand running 3'-->5'). If any two strands of nucleic acid (for example 2 strands of DNA in a double helix) are complementary (A-T/U or C-G), the two strands must run IN OPPOSITE directions, 5'--->3' and 3'--->5'. 2 If one strand of chromosome 2 has a DNA sequence that consists of this: 5’ AAG CGG TAC GTA 3’, what will be the composition of the complementary DNA strand? (Select all that apply) (1/1 Points) 5' TTC GCC ATG CAT 3' 3' TTC GCC ATG CAT 5' Correct! All complementary base pairing must be antiparallel. The strand that is complementary to 5’ AAG CGG TAC GTA 3’ is 3' TTC GCC ATG CAT 5'. If we simply 'flip' the sequence, we get 5' TAC GTA CCG CTT 3'. Thus, both of these are the correct answers. 3' TTC GCC ATG CAT 5' 3' TTC GCC ATG CAT 5' 5’ AAG CGG TAC GTA 3’ 3' AAG CGG TAC GTA 5' is similar to the original sequence (5’ AAG CGG TAC GTA 3’), but its 5' and 3' ends are swapped. 5' TAC GTA CCG CTT 3' Correct! All complementary base pairing must be antiparallel. The strand that is complementary to 5’ AAG CGG TAC GTA 3’ is 3' TTC GCC ATG CAT 5'. If we simply 'flip' the sequence, we get 5' TAC GTA CCG CTT 3'. Thus, both of these are the correct answers. 3' TTC GCC ATG CAT 5' 3' TTC GCC ATG CAT 5' 5’ AAG CGG TAC GTA 3’ 3' AAG CGG TAC GTA 5' is similar to the original sequence (5’ AAG CGG TAC GTA 3’), but its 5' and 3' ends are swapped. 3' AAG CGG TAC GTA 5' 5’ ATG TAC GGC GAA 3' 3 The following sequence is the coding strand of the collagen gene: 5’ ATG GCG TTC GAA 3’ What is the sequence of the corresponding mRNA? (0/1 Points) 3' ATG GCG TTC GAA 5' 5' AUG GCG UUC CUU 3' Incorrect. The coding strand and the mRNA both go in the same direction, but do not both contain Ts. 5' AUG GCG UUC GAA 3' 5' UTG GCG TTC GUU 3' 4 Which ingredients/molecules are required to set up a polymerase chain reaction? Select all that apply. (1/1 Points) Amino Acids RNA nucleotides DNA polymerase Correct! In PCR, a DNA sample is separated into single strands and incubated with DNA polymerase, deoxynucleotides (dNTPs), and two short DNA primers whose sequences flank the DNA segment of interest and thus define the region to be amplified. Recall from the section on DNA replication that DNA polymerase needs a primer to begin DNA synthesis. This requirement means the primers will direct the DNA polymerase to only synthesize complementary strands of the target DNA. DNA nucleotides Correct! In PCR, a DNA sample is separated into single strands and incubated with DNA polymerase, deoxynucleotides (dNTPs), and two short DNA primers whose sequences flank the DNA segment of interest and thus define the region to be amplified. Recall from the section on DNA replication that DNA polymerase needs a primer to begin DNA synthesis. This requirement means the primers will direct the DNA polymerase to only synthesize complementary strands of the target DNA. Template DNA Correct! In PCR, a DNA sample is separated into single strands and incubated with DNA polymerase, deoxynucleotides (dNTPs), and two short DNA primers whose sequences flank the DNA segment of interest and thus define the region to be amplified. Recall from the section on DNA replication that DNA polymerase needs a primer to begin DNA synthesis. This requirement means the primers will direct the DNA polymerase to only synthesize complementary strands of the target DNA. DNA Primers Correct! In PCR, a DNA sample is separated into single strands and incubated with DNA polymerase, deoxynucleotides (dNTPs), and two short DNA primers whose sequences flank the DNA segment of interest and thus define the region to be amplified. Recall from the section on DNA replication that DNA polymerase needs a primer to begin DNA synthesis. This requirement means the primers will direct the DNA polymerase to only synthesize complementary strands of the target DNA. Ribosomes RNA Primers 5 Which of the following steps in PCR occurs first? (1/1 Points) Elongation Annealing Cycling Denaturation Correct! Denaturation separated the DNA strands. The DNA strands must be separated to expose the bases to which the primers will bind. 6 Which statement accurately describes how a frameshift mutation affects a gene? (1/1 Points) It will change a single nucleotide in a mutant gene compared to the normal gene. Examples are missense, nonsense, and silent mutations. It will change the number of nucleotides in a mutant gene compared to the normal gene. Examples are missense, nonsense, and silent mutations. It will change a single nucleotide in a mutant gene compared to the normal gene. Examples are insertion and deletion mutations It will change the number of nucleotides in a mutant gene compared to the normal gene. Examples are insertion and deletion mutations. Correct! Background: Errors that change the number of nucleotides in a gene (insertion/deletion) are said to ‘shift’ the ‘reading frame’ from the correct groups of three basses to new groups. To visualize what a frameshift is, imagine you are given a string of letters and told to start at the beginning and read every group of three letters: CATDOGRATPIGAPE. You would get "cat dog rat pig ape". But if we add an extra letter, say CATDFOGRATPIGAPE, following our "read every group of three" rule, we would get "cat dfo gra tpi gap e". Shifting our 'reading frame' by adding one more letter completely changes what we read. This is similar to what happens with a frameshift mutation. 7 If the coding DNA sequence of a normal gene is 5' GTC GCA TGG TGA 3', what kind of mutation would create the mutant gene sequence 5' GTC GAC ATG GTG A 3'? (1/1 Points) Silent mutation Nonsense mutation Insertion mutation Correct! Background. An insertion mutation adds nucleotides to a gene and is a type of frameshift mutation, which changes the way the mRNA codons are read, changing the amino acid sequence. See Figure 1-19 in the Module 1 text for more information. Deletion mutation 8 A son with Tay Sachs was born into a family. What can you determine from the small family pedigree below? I. There isn't enough information; II. Sex-linked recessive is a possibility; III. Autosomal recessive is a possibility; IV. Sex-linked dominant is a possibility. (1/1 Points) II and III Correct! There is not enough information to know if it is X-linked or autosomal. To learn more about this please revisit the Lesson: DNA and RNA -> Chapter: Inheritance of Genes -> Subchapter: Inheritance -> Section: Representing the Inheritance of Alleles II only I only IV only 9 A black female mouse mates with a white male mouse and produces a litter of all gray mice. Which inheritance pattern can be used to describe this situation? (1/1 Points) Recessive Incomplete Dominance Correct! Incomplete dominance produces a blended phenotype. Complete Dominance Co-dominance 10 What do you think is the most likely mode of inheritance for hemophilia based on the pedigree below? (1/1 Points) Sex-linked dominant Autosomal dominant Autosomal recessive Sex-linked recessive Correct! The first generation contains unaffected parents having affected offspring, also known as carriers. Therefore, it must be recessive. Since only males are affected, it is X-linked recessive. 11 Some changes to the DNA do not modify the coding sequence of the DNA but do affect its winding and unwinding from nucleosomes. These changes can increase or decrease the availability of DNA and hence, the transcription of a gene. These are called _________ changes. (1/1 Points) Genetic Epigenetic Correct! Epigenetic changes result from modifications of the DNA that affect nucleosome spacing and, therefore, transcription. They do not alter the DNA sequence itself. Epidermal Hypogenetic 12 The LEP gene codes for an anorexigenic hormone. Interestingly, breastfeeding has been shown to alter methylation of the promoter of this gene, leading to increased LEP expression. What is likely happening in response to breastfeeding? (1/1 Points) Breastfeeding increases the methylation of the promoter of the LEP gene, decreasing the spacing between nucleosomes. Breastfeeding decreases the methylation of the promoter of the LEP gene, increasing the spacing between nucleosomes. Correct! When histones wrap up DNA, transcription will be repressed. Breastfeeding decreases the methylation of the promoter of the LEP gene, decreasing the spacing between nucleosomes. Breastfeeding increases the methylation of the promoter of the LEP gene, and the tight packing of the DNA alters binding of RNA polymerase. 13 A patient with xeroderma pigmentosum is prone to developing multiple skin cancers starting in childhood. This occurs because of a mutation in a gene that codes for enzymes that help repair DNA damage through the nucleotide excision repair (NER) pathways. How does NER differ from other repair mechanisms? In NER: (1/1 Points) Only one nucleotide is removed and DNA polymerase replaces the one abnormality The error in one strand of DNA is removed as well as several nucleotides on either side of the error. The gap that was removed is filled in by DNA polymerase Correct! The correct answer is that nucleotide excision repair replaces several damaged nucleotides plus additional nucleotides and uses the DNA on the opposite strand as a template to fill in the gap. See Figure 1-22 in the Module 1 text for more information. The entire homologous chromosome is used to repair the double stranded DNA error The affected DNA strand and its complementary strand is discarded and a new double stranded DNA is created 14 Several components of cigarette smoke, including benzopyrene, insert themselves (intercalate) into the DNA and lead to several types of mutations such as frameshift mutations, including both insertions and deletion. Which of the following repair pathways would be used to repair this type of damage? (1/1 Points) Nucleotide Excision Repair Correct! Nucleotide excision repair is used to repair deletions, insertions, and helix-distorting lesions, such as thymine dimers. To learn more about this, please visit Lesson 1: DNA and RNA; Chapter - Inheritance of Genes; Subchapter - DNA Damage and Repair; Section - Excision Repair Corrects The Most Frequent DNA Lesions. Base excision repair Homologous Recombination Mismatch Repair 15 What bond is indicated by the arrow in the diagram? (0/1 Points) Ionic bond Peptide bond Hydrogen bond Metallic bond Incorrect. If you answered "metallic bond" you are incorrect. This type of bond is not covered in this course. 16 Interaction between the two amino acids shown below can help stabilize the structure of a protein. What kind of interaction is this? (0/1 Points) Hydrogen bond Hydrophobic interaction Ionic bond If you selected Ionic, you are incorrect. Amino acids with opposite charged R groups will be attracted to each other and form an ionic bond. Disulfide bond 17 Which pair of amino acids below can form an ionic bond? (1/1 Points) Option 1 Option 2 Option 3 If you picked option 3, you are correct! Amino acids with side chains that are oppositely charged can form ionic bonds. The charges on charged amino acids are visibly displayed on the R group of the individual amino acids. Option 4 18 Which of the following forces can lead to aggregation as a result of protein misfolding? (1/1 Points) hydrophobic interactions If you selected hydrophobic interactions, you are correct. When proteins misfold, their hydrophobic amino acids at their cores are exposed to the aqueous environment in the cell. To avoid water, the hydrophobic/nonpolar amino acids that have been exposed will interact with other hydrophobic/nonpolar amino acids, causing aggregation (the linking of proteins together in clumps). hydrogen bonds disulfide bonds ionic bonds 19 Which of the following statements about protein structure and stability is true? (1/1 Points) Denatured proteins retain their tertiary structure Ionic bonds between the side chains of the charged amino acids stabilize the protein structure Correct! The true statement is "Ionic bonds between the side chains of the charged amino acids stabilize the protein structure." Ionic bonds help stabilize both the tertiary structure of a protein chain and the quaternary structure of a protein with multiple subunits. Please see "R Group Contributions to Protein Structure and Stability" in Module 2, Lesson 1 under "Levels of Protein Structure" for more information. Denaturation is the loss of primary, secondary, and tertiary structure Protein structure is not stabilized by the hydrophobic effect 20 Primary structure consists of the order of ______ in a protein. These are held together with ______ bonds that are formed by a ______ reaction. (1/1 Points) Nucleotides, phosphodiester, dehydration Amino acids, peptide, hydrolysis Amino acids, peptide, dehydration Correct! Protein primary structure is defined by the order of amino acids that make up the protein. The amino acids are linked together by peptide bonds, which are formed via dehydration reactions. For more information, please see "Peptide Bonds Link Amino Acids in Proteins" and "The Amino Acid Sequence is the First Level of Protein Structure" in the section "Amino Acids and Primary Structure" of Module 2, Lesson 1. Nucleotides, peptide, dehydration 21 What type of reaction breaks peptide bonds apart? (1/1 Points) Methylation Reaction Oxidation/Reduction Reaction Hydrolysis Reaction Correct! Peptide bonds are formed by dehydration reactions (named for the loss of water that occurs) and broken via hydrolysis (named for the addition of water: "hydro-" meaning water, and "-lysis" meaning cutting, i.e. water cuts the peptide bond). Condensation Reaction 22 A particular amino acid in a protein helps to stabilize the protein by forming disulfide bonds. What amino acid is likely to form this type of bond? (1/1 Points) Cysteine Correct! Cysteine is the only amino acid with -SH and is, therefore, the only amino acid that can form disulfide bonds. Methionine Either Cysteine or Methionine Neither Cysteine nor Methionine 23 Aggregation of proteins is the main reason behind many neurodegenerative diseases. Which one of the following mutations will likely cause a neurodegenerative disease? (1/1 Points) Replacing a positively charged amino acid with a negatively charged amino acid Replacing a polar amino acid with a non polar amino acid Correct! Replacing a polar amino acid with a nonpolar amino acid will lead to protein misfolding. Replacing a polar amino acid with another polar amino acid Replacing a negatively charged amino acid with a negatively charged amino acid 24 Chymotrypsin is a digestive enzyme that has optimal activity at a pH of 7.8 and a temperature of 37 degrees Celsius. Which of the following statements is true? (1/1 Points) At a temperature of 100 degrees Celsius, chymotrypsin will have slight activity. At a pH of 8.5, chymotrypsin will have increased activity compared to chymotrypsin at a pH of 7.8. At a pH of 8.5, chymotrypsin will have decreased activity compared to chymotrypsin at a pH of 7.8. Correct! Chymotrypsin has an optimal pH of 7.8 and optimal temperature of 37 degrees Celsius. Anything outside of that range will decrease the activity of chymotrypsin. Since pH 8.5 is outside of that range, chymotrypsin would have less activity. At a temperature of 20 degrees Celsius, chymotrypsin will demonstrate increased activity compared to a reaction at 37 degrees C. 25 A patient presents with a fever of 110°F. Which interaction(s) would be disrupted within a neuronal protein if the fever is not resolved quickly? (1/1 Points) Disulfide Bond Hydrogen Bond Ionic Bonds Hydrophobic Interactions Correct! The patient's temperature is much higher than normal. High temperatures disrupt hydrophobic interactions. 26 A diabetic patient is suffering from ketoacidosis. Which interaction(s) could be disrupted within the patientʼs hemoglobin due to this condition. Select all that apply. (1/1 Points) Hydrogen Bond Correct! Diabetic ketoacidosis leads to a lower pH than normal. Changes in pH can disrupt both hydrogen bonds and ionic bonds inside of a protein. Ionic Bonds Correct! Diabetic ketoacidosis leads to a lower pH than normal. Changes in pH can disrupt both hydrogen bonds and ionic bonds inside of a protein. Hydrophobic Interactions Disulfide bond 27 Which of the following forces can lead to aggregation as a result of protein misfolding? (1/1 Points) hydrogen bonds ionic bonds hydrophobic interactions Correct! Misfolded proteins can have sections of hydrophobic amino acid residues exposed to water. This can lead to misfolded proteins to aggregate in order to form favorable hydrophobic interactions between nonpolar amino acids in adjacent protein chains and to keep these hydrophobic residues away from water. disulfide bonds 28 Which of the following are true about a misfolded protein? Select all that apply. (1/1 Points) It can cause protein aggregation. Correct! "It can cause protein aggregation:" Misfolded proteins can have sections of hydrophobic amino acid residues exposed to water. This can lead to misfolded proteins to aggregate in order to keep these hydrophobic residues away from water. It loses its primary structure. It can be degraded by the cell. Correct! "It can be degraded by the cell:" In addition to chaperone proteins being able to help misfolded protein refold, the cell also has mechanisms for identifying and breaking down misfolded proteins into amino acids to prevent these misfolded proteins from forming aggregates. It will lose its normal function. Correct! "It will lose its normal function:" The function of a protein is heavily dependent on its three-dimensional shape. As a protein misfolds changes in three-dimensional shape lead the protein to lose its ability to perform its normal function. It can be the result of denaturation. Correct! "It can be the result of denaturation:" Denaturation disrupts side chain interactions, which destabilizes tertiary structure and can cause the protein to change shape, i.e. misfold. 29 Which of the following accurately describes the functions of hemoglobin and myoglobin? (1/1 Points) Hemoglobin stores oxygen, Myoglobin transports oxygen. Hemoglobin transports CO2 and Myoglobin stores CO2 Hemoglobin transports oxygen, Myoglobin stores oxygen. Correct! Myoglobin transports CO2 and Hemoglobin stores CO2 30 Which of the following statements accurately describes cooperativity? (1/1 Points) Oxygen and carbon dioxide exchange occurs in the alveoli of the lungs. Myoglobin and hemoglobin work together to deliver oxygen to the tissues. Hemoglobin can bind subsequent molecules of oxygen more easily after the first oxygen molecule binds. Correct! Myoglobin can bind subsequent molecules of oxygen more easily after the first oxygen molecule binds. 31 Carbon monoxide is poisonous because the ability of carbon monoxide to bind to hemoglobin is ___________ than the ability of oxygen to bind to hemoglobin. (1/1 Points) 20 times lower 200 times lower 20 times higher 200 times higher Correct! Carbon monoxide is toxic because hemoglobin prefers to bind carbon monoxide over oxygen. This is due to hemoglobin's increased affinity for carbon monoxide. Hemoglobin's affinity for carbon monoxide is 200 times greater than hemoglobin's affinity for oxygen. The correct answer choice is "200 times greater". 32 Patients with carbon monoxide (CO) poisoning may exhibit pink or cherry red skin, mucus membranes and nails. Which statement accurately describes hemoglobin's role in CO poisoning? (1/1 Points) Hb binds to CO with a lower affinity than oxygen and stabilizes the T-state. Hb binds to CO with a lower affinity than oxygen and stabilizes the R-state. Hb binds to CO with a higher affinity than oxygen and stabilizes the R-state. Correct! Hemoglobin binds 200-times more strongly to CO than oxygen and When CO binds to hemoglobin, it stabilizes the R-state of the protein. The R state of hemoglobin is responsible for the pink or cherry red color observed in CO poisoning. The correct answer choice is "Hemoglobin binds CO with a higher affinity than oxygen and stabilizes the R-state." Hb binds to CO with a higher affinity than oxygen and stabilizes the T-state. 33 In which of the following patients is the planar conformation of the heme group in hemoglobin favored? (1/1 Points) In an older woman with pneumonia and sepsis who has a blood pH of 7.1 In a firefighter who is brought to the emergency room after entering a burning building Correct! The planar conformation of heme would be favored under conditions of high pH (at high pH, hemoglobin prefers to bind oxygen and heme is in the planar conformation). A hospitalized firefighter would presumably be receiving oxygen and would thus have more hemoglobin in the oxygen binding conformation. The correct answer choice is "In a firefighter who is brought to the emergency room after entering a burning building". In a girl with diabetic ketoacidosis In the muscle capillaries of a patient who is on a treadmill for a cardiac stress test 34 Below is a picture of the hemoglobin dissociation curve at different pH levels. Which curve demonstrates the saturation of hemoglobin in the lungs? (0/1 Points) pH 7.4 If you chose "pH 7.4", the answer is incorrect. Compared to the other two curves, the pH 7.4 curve has a moderate affinity for oxygen, and represents normal blood pH. For example, at 20 torr, ~35% of hemoglobin is bound to oxygen at pH 7.4 compared to only ~20% at pH 7.2 and ~50% at pH 7.6. Hemoglobin in the lungs needs to have a high affinity for oxygen so that it can bind to oxygen in the lungs and carry it throughout the body. The Bohr effect says that as the pH increases, the affinity of hemoglobin for oxygen increases, so we would expect the lungs to be represented by the curve with the highest affinity = the highest pH = pH 7.6. See "The Bohr Effect Enhances Oxygen Transport" in Module 3 for more information." in Module 3 for more information. pH 7.2 pH 7.6 35 The Bohr effect is a relationship between hemoglobin's oxygen binding behavior in conjunction with the pH of the surroundings. Which of the following statements describes hemoglobin at HIGH pH? (0/1 Points) When the pH is high, hemoglobin has a high affinity for oxygen and releases oxygen. Incorrect. If you chose "When the pH is high, hemoglobin has a high affinity for oxygen and releases oxygen", the answer is incorrect. The Bohr effect states that as the pH increases, the affinity of hemoglobin for oxygen also increases. Therefore, the "high affinity" part of the answer is true, but when the affinity is high, hemoglobin binds, not releases, oxygen. See "The Bohr Effect Enhances Oxygen Transport" in Module 3 for more information. When the pH is high, hemoglobin has a high affinity for oxygen and binds oxygen. When the pH is high, hemoglobin has a low affinity for oxygen and binds oxygen. When the pH is high, hemoglobin has a low affinity for oxygen and releases oxygen. 36 The product of a 4-step pathway accumulates and inhibits the first enzyme in the pathway. This is an example of ________ inhibition. (1/1 Points) Mixed Competitive Uncompetitive Feedback Correct. Feedback inhibition results when the end product of a pathway inhibits the first enzyme of a pathway. 37 When inhibitor F is present, which best describes what will happen to enzyme 2? (1/1 Points) Enzyme 2 will be inactivated due to low levels of molecule D. Enzyme 2’s activity will be high due to negative feedback. Enzyme 2’s activity will be low due to negative feedback. The inhibition of enzyme 2 will be relieved. Correct. The inhibition of Enzyme 2 will be relieved. When Inhibitor F is present, Enzyme 1 is inhibited. With this enzyme inhibited, levels of Molecules B, C, and D decrease. When Molecule D decreases, it no longer inhibits Enzyme 2 through feedback inhibition. Although its substrate levels will be low, Enzyme 2 will have its inhibition relieved. 38 How do enzymes eliminate the need for high temperatures to complete a reaction? (1/1 Points) The need for high temperatures is eliminated by increasing the thermal energy needed for the reaction The need for high temperatures is eliminated by increasing the activation energy needed for the reaction The need for high temperatures is eliminated by increasing the kinetic energy needed for the reaction The need for high temperatures is eliminated by lowering the activation energy needed for the reaction Correct! Kinetic energy is the energy of motion. When heat is added to a reaction, it causes the particles to move faster, and the temperature typically increases. The presence of the enzyme actually lowers the amount of heat needed for the reaction, or lowers the amount of kinetic energy needed for the reaction to occur. 39 How do enzymes increase the rate of a reaction? (1/1 Points) Decreasing the activation energy of a reaction. Correct! The enzyme brings the right amino acids into the right locations so that the enzyme can bind the substrate and allow the substrate to change into product. Since the groups are in the right places, less energy needs to be added to get the reaction over the energy hill. This results in a decrease in the activation energy and a faster reaction. Stabilizing the product of the reaction Increasing the temperature of a reaction Increasing the activation energy of a reaction 40 Which of the following factors can affect the protein folding and activity of an enzyme? (1/1 Points) pH All of the options are correct Correct! Heat can disrupt hydrophobic interactions at the center of the protein. pH can disrupt ionic interactions, and also hydrogen bonds. Reducing agents can break disulfide bonds. All of these changes could affect the activity of the enzyme. Heat Reducing agents 41 The enzyme Acetylcholinesterase breaks down acetylcholine in the brain leading to neuron degeneration. Which of the following strategies represents the best way to increase acetylcholine levels? (1/1 Points) Increase gene expression for acetylcholinesterase. Allosteric activation of acetylcholinesterase. Competitive inhibition of acetylcholine. Non-competitive inhibition of acetylcholinesterase. Correct! Non-competitive inhibition of acetylcholinesterase. If acetylcholinesterase is inhibited, then the substrate, acetylcholinesterase, will increase. If the inhibitor is a competitive inhibitor, the substrate levels will eventually overcome the inhibition, which will be relieved. If the inhibitor is a non-competitive inhibitor, the concentration of substrate does not affect the inhibition. Therefore, non-competitive inhibition of acetylcholinesterase is the best approach. 42 42) In the reaction provided below, glucose-6-phosphate accumulates in the cell and binds to hexokinase at a site that is distinct from the active site. This prevents hexokinase from binding to its substrate. What type of inhibition is occurring in this example? (1/1 Points) anti-competitive inhibition Non-competitive inhibition Correct! Non-competitive. Glucose-6-phosphate binds to a site different than the active site, indicating that it is binding an allosteric site. When an allosteric site is bound by an inhibitor, the active site is altered, preventing substrate binding. This is the definition of non-competitive inhibition. Competitive inhibition 43 In Chronic Mylogenous Leukemia (CML), a mutation results in the production of BCR-ABL, an enzyme that speeds up cell division using ATP as a substrate. The drug Gleevec, impairs the activity of the enzyme. Using the illustrations of the enzyme below, what best describes how the drug exerts its effect? (0/1 Points) Gleevec is a competitive inhibitor and prevents ATP from binding with the active site of the enzyme. Gleevec is a competitive inhibitor that binds to an allosteric site on the enzyme to prevent ATP from binding to the active site. Incorrect. Gleevec is a competitive inhibitor and prevents ATP from binding with the active site of the enzyme.Competitive inhibitors bind to an enzyme in the active site. Gleevec is binding in the same site as ATP. ATP is the substrate, to the site of ATP binding must be the active site. Gleevec is therefore is binding the active site and is a competitive inhibitor. If you chose, "Gleevac is a non-competitive inhibitor..." this is incorrect because non-competitive inhibitors bind at a site different than the active site. In the figure it can be seen that Gleevac binds the same site on the enzyme as the substrate (ATP). This means that Gleevac is binding the active site. If you chose "Gleevac is a competitive inhibitor that binds to an allosteric site...", this is incorrect because competitive inhibitors bind the active site, not an allosteric site. Gleevec is a non competitive inhibitor and prevents ATP from binding with the active site of an enzyme. Gleevec is a non competitive inhibitor that binds to an allosteric site on the enzyme to prevent ATP from binding to the active site. 44 What happens to the enzyme at the end of the cycle? (1/1 Points) The enzyme is used again. The enzyme will bind to a substrate. Correct! During the process of changing the substrate into product and product release, the amino acids in the active site where the chemistry occurred on the substrate are changed back into their original state. This means that, when the product is formed and then released, the same amino acids will then be available to accept the next substrate molecule and repeat the same chemistry. A catalyst, in general, is a substance that speeds up a reaction by lowering the activation energy for the reaction. It is also unchanged, overall, from the form it was in at the start of the reaction. This means that it can be used over and over again. Enzymes are special protein catalysts that accept specific substrates and turn them into products, and are used in an incredible number of chemical reactions in living organisms. Remains bound to the product. The enzyme is destroyed. The enzyme shape is altered and it cannot be re-used for another reaction. 45 Inhibitors that have a similar structure to a substrate of an enzyme are most likely to bind to the enzyme’s ____________ and be a ____________ inhibitor. (0/1 Points) Active site, Competitive Allosteric site, Competitive Allosteric site, Non-competitive Incorrect. The correct answer is 'Active site, Competitive' If you chose 'allosteric site, non-competitive', this is incorrect because the allosteric site is usually bound by inhibitors that have a different shape than the substrate and inhibitors that bind the active site are called competitive inhibitors. If you chose 'active site, non-competitive' this is incorrect because inhibitors that bind the active site are called competitive inhibitors. If you chose 'allosteric site, competitive', this is incorrect because the allosteric site is usually bound by inhibitors that have a different shape than the substrate. Useful information for all choices: Since competitive inhibitors are able to bind the active site of the enzyme in place of the substrate, competitive inhibitors often have a similar structure to the substrate. Active site, Non-competitive 46 You are in charge of designing a drug that inhibits the activity of a specific enzyme. An important criteria for the drug selection is to ensure that the drug directly competes with the original substrate by binding to the active site of the enzyme. Which of the following kind of inhibitor would be an ideal choice? (0/1 Points) Uncompetitive inhibitor Non-specific inhibitor Non-competitive inhibitor Incorrect. The correct answer is 'Competitive inhibitor.' If you chose 'non-competitive', this is incorrect because non-competitive inhibition involves binding of the inhibitor to a site different than the active site and the question asks for a type of inhibitor that binds the active site. Useful information for all choices: A competitive inhibitor is usually a molecule similar in structure to a substrate that can bind to an enzyme’s active site even though the molecule is unable to react. This non-substrate molecule competes with the substrate for the active site. When the inhibitor binds to an active site, it prevents the substrate from binding and thereby inhibits the reaction. Competitive inhibitor 47 What process takes place in the inner membrane of the mitochondria? (1/1 Points) electron transport chain Correct! The electron transport chain occurs in the inner membrane of the mitochondria beta oxidation glycolysis lactate fermentation 48 Where is energy stored in an ATP molecule? (1/1 Points) In the bond between the adenosine molecule and the 1st phosphate In the adenine nitrogenous base portion of the adenosine molecule. In the bonds between the phosphate groups. Correct! The bonds between the phosphate groups are very high in energy. Adenosine triphosphate (ATP) contains three, high energy phosphate groups attached to an adenosine molecule. The phosphate bond with the greatest amount of energy is between the last phosphate (gamma) and the second phosphate (beta) In the sugar portion of the adenosine molecule 49 Which of the following processes does NOT take place in the mitochondria of a cell? (0/1 Points) Production of CO2 in the citric acid cycle Incorrect. If you chose the option 'Production of CO2 in the citric acid cycle', the answer is incorrect. The citric acid cycle occurs in the matrix of the mitchondria. One of the products the citric acid cycle is CO2. If you chose the option 'Production of FADH2', the answer is incorrect. FADH2 is a product of the citric acid cycle, which occurs in the matrix of the mitochodria. If you chose the option 'Creation of a proton gradient', the answer is incorrect. The proton gradient is generated in the electron transport chain, which occurs in the inner membrane of the mitochondria. The correct answer is 'Conversion of pyruvate to lactic acid'. Production of FADH2 Conversion of pyruvate to lactic acid Creation of a proton gradient 50 Carbohydrates, fatty acids, and amino acids can all enter the citric acid cycle through which molecule? (1/1 Points) Pyruvate Acetyl-CoA Correct! Carbohydrates, fatty acids, and amino acids all enter the citric acid cycle through acetyl-CoA. ATP NADH 51 During strenuous exercise, oxygen is scarce in skeletal muscle fibers. How many net ATP molecules are produced during glycolysis? (1/1 Points) 30 2 Correct! Glycolysis creates 4 ATP, but 2 ATP are consumed during the pathway so there is a net gain of 2 ATP -6 4 52 A defect occurs that prevents pyruvate from converting to lactate under anaerobic conditions. What is a possible consequence? (1/1 Points) Glycolysis stops because NAD+ levels are low Yes! When pyruvate is converted to lactate under anaerobic conditions, NAD+ is regenerated from NADH; NAD+ is essential for glycolysis to occur. Glycolysis continues because NAD+ levels are low Glycolysis stops because NAD+ levels are high Glycolysis continues because NAD+ levels are high 53 How many rounds of beta oxidation are needed to break down the pictured fatty acid? (0/1 Points) 8 7 17 18 Incorrect. It requires 8 rounds of beta oxidation to produce 9 acetyl-CoA from an 18-carbon fatty acid. ((18/2)-1) = 8. 54 The fatty acid CH3(CH2)4CH=CH(CH2)4COOH has been through one round of beta oxidation. What is the structural formula for the remaining fatty acid? (1/1 Points) CH3(CH2)2CH=CH(CH2)4COOH CH3(CH2)8COOH CH3(CH2)4CH=CH(CH2)2COOH Correct! After CH3(CH2)4CH=CH(CH2)4COOH (C12 fatty acid) has undergone one round of beta oxidation, the product is CH3(CH2)4CH=CH(CH2)2COOH (C10 fatty acid); the fatty acid contain 2 fewer carbons at the carboxyl end. Note that the beta bond that is oxidized during beta oxidation is two carbon atoms in from the carboxyl end of the fatty acid. Beta oxidation is the pathway used to breakdown fatty acids so they can be used to create ATP. Each time a fatty acid undergoes beta oxidation, a 2-carbon fragment is removed from the carboxyl (COOH) end of the fatty acid; this fragment is called acetyl-CoA. Acetyl-CoA can then enter the citric acid cycle. CH3(CH2)4CH=CHCH2COOH 55 In the absence of oxygen in the exercising muscle, why does the amount of lactate in the blood change? (1/1 Points) The amount of lactate does not change in the absence of oxygen in exercising muscles. When oxygen is not present, muscles use lactate to make ATP through the glycolysis pathway.. Lactate decreases since it is produced by metabolizing muscle but has no other use in the body. The amount of lactate increases because lactate produced in muscles anaerobically must travel to the liver. Correct! Lactate produced during anaerobic metabolism leaves the muscle cells and travels to the liver via the blood. 56 How does the change in lactate concentration affect the pH of the blood in muscle tissue? (1/1 Points) The pH of the blood in the muscle tissue first increases and then immediately decreases since the lactate is soon used by the muscle. Lactate causes the pH of the blood to increase because lactate is an acid. Lactate has no effect on pH since pH measures oxygen concentration in the blood. An increase in lactate concentration causes the pH of the blood in muscle tissue to decrease. Correct! Lactate is an acid (lactic acid) and therefore leads to a decrease in pH. 57 Which of the following differentiates aerobic from anaerobic cellular metabolism? (1/1 Points) The production of ATP The breakdown of glucose into compound with fewer carbon atoms The utilization of O2 as an electron acceptor Yes! Only aerobic metabolism depends on oxygen as the terminal electron acceptor in the electron transport chain. The use of ingested food to create energy 58 How does glucagon increase blood glucose concentration? (1/1 Points) Glucagon stimulates fatty acid synthesis Glucagon stimulates glycogenesis Glucagon stimulates gluconeogenesis and glycogenolysis Correct! Glucagon stimulates gluconeogenesis which creates new glucose molecules from non carbohydrate sources and by glycogenolysis which breaks down the glycogen to individual glucose units. Glucagon acts on hepatocytes (liver cells) to accelerate the conversion of glycogen into glucose (glycogenolysis) and to promote formation of glucose from lactic acid and certain amino acids (gluconeogenesis). Glucagon stimulates beta-oxidation 59 59) Hemoglobin A1c (sometimes referred to simply as A1c) is a molecule measured for a laboratory test. It is used to confirm the diagnosis of diabetes and to assess a diabetic patient’s level of disease control. How and why do advanced glycation end products (AGEs) form? (1/1 Points) Low blood glucose causes glycation, a reaction where an ionic bond forms between a sugar molecule and a nucleic acid. Additional glycation reactions cross-link proteins together into AGEs, which can impair the function of the proteins and their associated organs. High blood glucose causes phosphorylation, a reaction where a covalent bond forms between a sugar molecule and a monosaccharide. Additional glycation reactions cross-link proteins together into AGEs, which can impair the function of the proteins and their associated organs. High blood glucose causes glycation, a reaction where a covalent bond forms between a sugar molecule and a protein. Additional glycation reactions cross-link proteins together into AGEs, which can impair the function of the proteins and their associated organs. Correct! High blood glucose causes glycation, a reaction where a covalent bond forms between a sugar molecule and a protein. Additional glycation reactions cross-link proteins together into AGEs, which can impair the function of the proteins and their associated organs. Glycation is the reaction in which a covalent bond forms between a sugar molecule and a protein or lipid molecule without the aid of an enzyme. The addition of the sugar can affect the function of a protein by making it more stiff and inflexible. Glycation can also lead to additional reactions that cross-link proteins together into advanced glycation end products (AGEs), which can impair the function of the proteins and their associated organs. 60 A woman is about to begin her ascent of Mount Everest and is preparing for Day 1 of the climb. Approximately 6 hours before the hike begins, she and her climbing team eat a meal containing 80% complex carbohydrates. What is the physiological benefit of this approach? (1/1 Points) Increasing glycolysis in the hours leading up to the hike Stimulating the pancreas to release glucagon Raising insulin levels Improving glycogen stores in liver and muscle Correct! Improving glycogen stores in liver and muscle. Glycogen is a stored form of glucose. Individual glucose units are polymerized to form glycogen and is stored in liver and muscle. People who partake in endurance sports consume complex carbohydrate meals right before the activity or sport in order to improve their glycogen stores in liver and muscle. During the activity, the stored glycogen is then broken down to supply the much needed glucose to sustain the athlete. 61 For the image below, which option correctly identifies the part of the fatty acid indicated by C? (1/1 Points) Omega Carbon Beta Carbon Correct! C is pointing to the beta carbon. A- omega carbon, B- alpha carbon, D- beta bond. Alpha Carbon Beta Bond 62 CH3(CH2)4CH=CH(CH2)6COOH is an example of which type of fatty acid? (check all that apply) (1/1 Points) Polyunsaturated fatty acid Saturated fatty acid Cholesterol Omega-6 fatty acid Correct! The structure represents a fatty acids with an omega 6 double bond between the carbons in the hydrocarbon chain. Unsaturated fatty acids have at least one double bond between the carbons in the hydrocarbon chain. It is not a saturated fatty acid because saturated fatty acids do not have any double bonds between the carbons in the hydrocarbon chain. It is not cholesterol because cholesterol has a tetracyclic (tetra means four) ring structure. Unsaturated fatty acid Correct! The structure represents a fatty acids with an omega 6 double bond between the carbons in the hydrocarbon chain. Unsaturated fatty acids have at least one double bond between the carbons in the hydrocarbon chain. It is not a saturated fatty acid because saturated fatty acids do not have any double bonds between the carbons in the hydrocarbon chain. It is not cholesterol because cholesterol has a tetracyclic (tetra means four) ring structure. 63 Which of the following is an example of an essential fatty acid? (check all that apply) (1/1 Points) CH3(CH2)8CH=CH(CH2)4COOH CH3CH2CH=CH(CH2)4COOH Correct! You selected all three of the correct answers. Essential fatty acids contain an omega 3 and/or omega 6 double bond(s) within the carbons in the hydrocarbon chain. CH3CH2CH=CHCH2CH=CH(CH2)4COOH Correct! You selected all three of the correct answers. Essential fatty acids contain an omega 3 and/or omega 6 double bond(s) within the carbons in the hydrocarbon chain. CH3(CH2)10COOH CH3(CH2)4CH=CH(CH2)6COOH Correct! You selected all three of the correct answers. Essential fatty acids contain an omega 3 and/or omega 6 double bond(s) within the carbons in the hydrocarbon chain. 64 Saturated fatty acids have more _________________ than unsaturated fatty acids of the same length. (1/1 Points) Carbon atoms Oxygen atoms Hydrogen atoms Correct! Saturated fatty acids contain more hydrogen atoms than unsaturated fatty acids. Saturated fatty acids do not have double bonds between the carbons within the hydrocarbon chain. Fatty acids have the same amount of oxygen atoms, two. These oxygen atoms are found within the carboxylate group. Both saturated and unsaturated fatty acids can have up to 24 carbons. double bonds 65 Which lipid has a 4-ring structure? (0/1 Points) Phospholipid Incorrect: Plasminogen, fatty acids, and phospholipids do not have a tetracyclic ring. The correct answer is sterols. Sterols have a tetracyclic ring (four ring structure). Fatty Acid Plasminogen Sterol 66 Lipases are enzymes that break down triglycerides into ________ and three free fatty acids. (1/1 Points) Sterols Glucose Glycerol Correct! Glycerol is part of a triglyceride. Sterols, glucose, and vitamins are not constituents of a triglyceride molecule. Vitamins 67 Contains a kink that makes packing difficult: (1/1 Points) Saturated fatty acid Unsaturated fatty acid Correct! Cis-unsaturated fatty acids have a bend/kink in their structure. Cholesterol Ketone 68 The cellular membrane consists of a lipid bilayer made up of __________. (1/1 Points) Eicosanoids Phospholipids Correct! Phospholipids are a major component of cell membranes. Triglycerides Saturated fatty acids 69 Fatty acid chains can range anywhere from 4 to 36 carbons. When enzymes that are specific for breaking down a particular length of fatty acid chain are defective, fatal diseases occur. Which of the following diseases is associated with failure to breakdown fatty acids? (1/1 Points) MCADD (Medium-chain acyl-CoA dehydrogenase deficiency) Correct! MCADD is a disease that results from a defect in the beta oxidation of medium-chain fatty acids. MCADD usually presents in infants as vomiting and/or lack of energy due to their inability to utilize medium-chain fatty acids. Sickle cell disease Alzheimer's disease Hemophilia 70 During starvation, triglycerides can help supply some of the body’s glucose needs. Which breakdown product of triglycerides can be used to make glucose? (1/1 Points) Ketone bodies Acetyl-CoA Glycerol Correct! The glycerol portion of a triglyceride can be converted to pyruvate and used to make glucose via gluconeogenesis. Cholesterol [Show More]
Last updated: 2 years ago
Preview 1 out of 24 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 10, 2021
Number of pages
24
Written in
All
Additional information
This document has been written for:
Uploaded
Mar 10, 2021
Downloads
0
Views
182

.png)