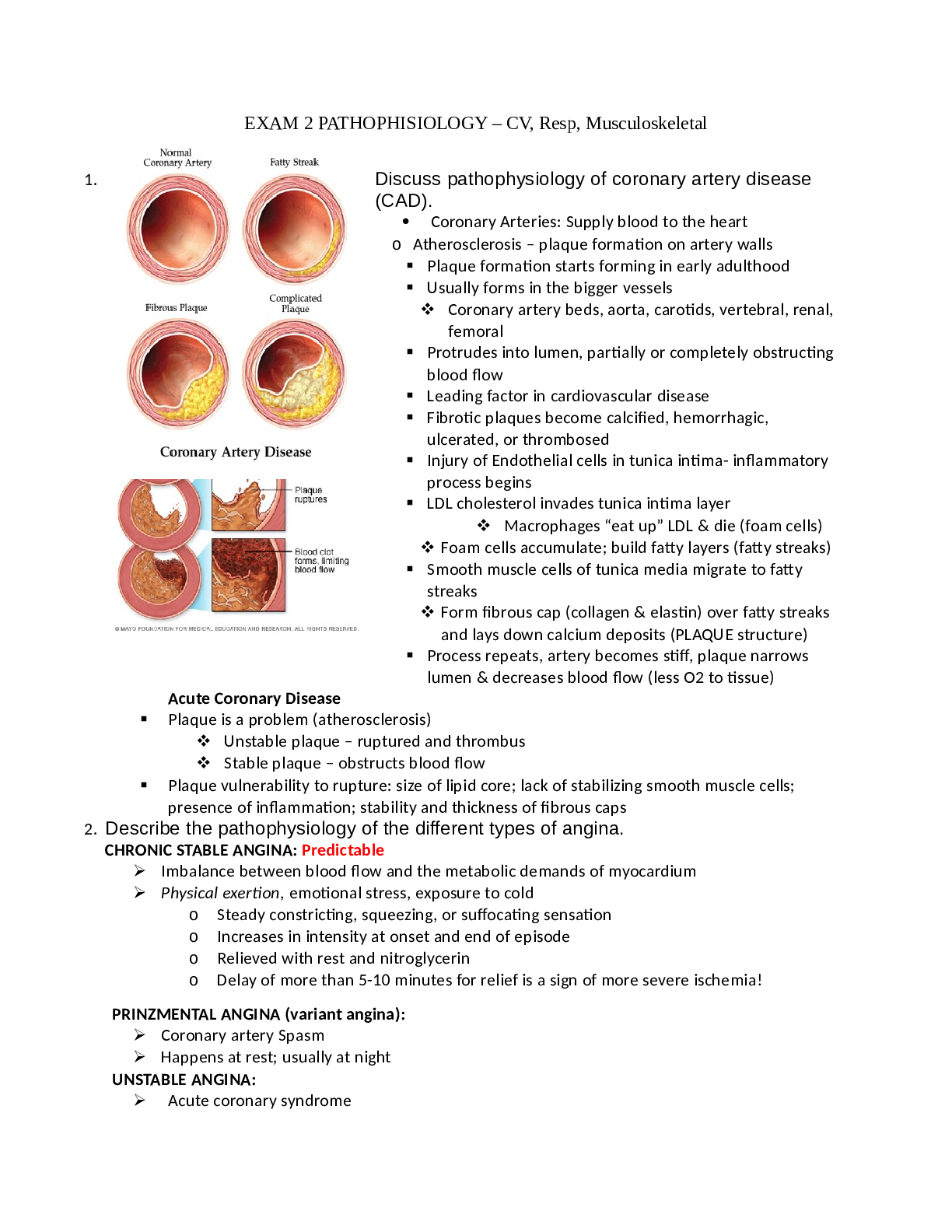
Pearson Edexcel_A Level Physical Education_9PE0/02 Mark Scheme 2020 | Psychological and Social Principles of Physical Education
$ 6.5

Gizmo Student Exploration: Building DNA, (A Grade), Questions and Answers, All Correct Study Guide
$ 14
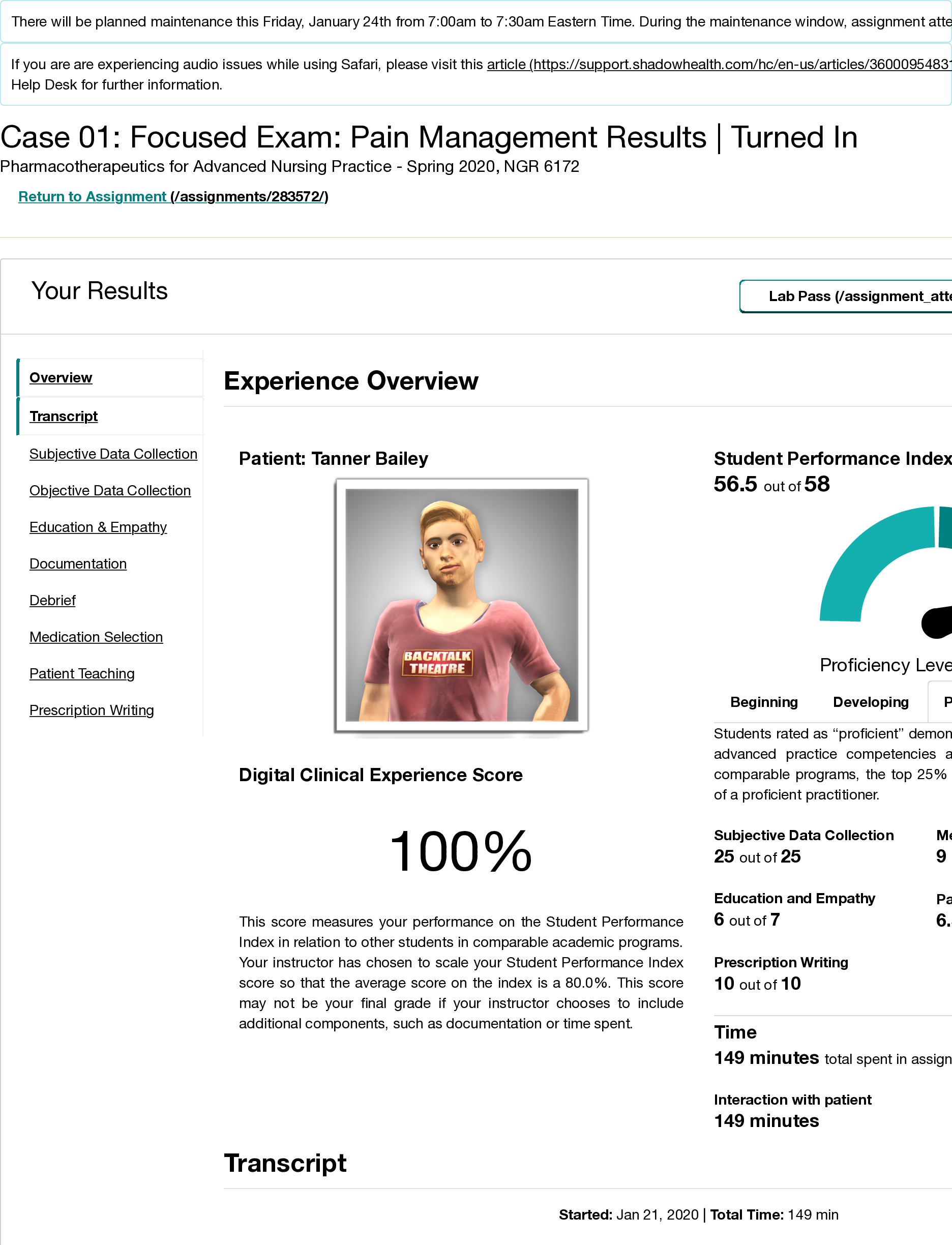
Tanner Bailey Pain Management Shadow Health Exam- Education and Empathy (Tanner Bailey)
$ 14.5

MEDICAL EMERGENCIES FISDAP QUESTIONS 3rd test
$ 11.5

ACCT-346 Week 2 Discussion: Job-Order Costing (GRADED)
$ 13.5

Pearson Edexcel_History_8HI0/1F Mark Scheme_2020 | Paper 1: Breadth study with interpretations
$ 6.5

Pearson Edexcel A-level Economics Unit 1 Complete Revision Notes(WEC11)
$ 18

IHUMAN CASE BETTY BURNS PERFORMANCE OVERVIEW
$ 15

EXAM 2 STUDY GUIDE HEMATOLOGIC DISORDERS: 7-10 questions
$ 14
.png)
Pearson Edexcel Merged Question Paper + Mark Scheme (Results) Summer 2022 Pearson Edexcel GCSE In French (1FR0) Foundation Tier Paper 03: Reading and Understanding in French
$ 6

Bioresources and Bioprocess in Biotechnology for a Sustainable Future 1st Edition by Leonardo Sepúlveda Torre, Juan Carlos Contreras-Esquivel, Ann Rose Abraham, A. K. Haghi
$ 30

> GCE Mathematics B (MEI) H640/03: Pure Mathematics and Comprehension Advanced GCE Mark Scheme for Autumn 2021
$ 6

eBook Suprapontine Lesions and Neurogenic Pelvic Dysfunctions Assessment, Treatment and Rehabilitation 1st edition by Gianfranco Lamberti, Donatella Giraudo, Stefania Musco
$ 13

AVIA 245 Quiz 1 With Correct Answers. Latest 2022
$ 10
.png)
Pearson Edexcel GCE Question Booklet + Mark Scheme (Results) November 2021 In History (9HI0) Paper 1: Breadth study with interpretations Paper Option 1A: The crusades, c1095–1204
$ 10
 Complete Answer key 2022.png)
GIZMOS -Free-Fall (SE) Complete Answer key 2022
$ 10

AQA GCSE COMBINED SCIENCE: TRILOGY Higher Tier Biology Paper 1H QP 2020
$ 12.5

marvin webster case study - Walden University
$ 10

HESI Med Surg Questions and Answers 100% Correct
$ 10

Unit_4._Blog_About_It University of Minnesota-Twin Cities BBE 1002
$ 4

AQA A-level PHYSICAL EDUCATION 7582/2 Paper 2 Factors affecting optimal performance in physical activity and sport Mark scheme June 2021 Version: 1.0 Final Mark Scheme
$ 10

Nursing Quiz
$ 8
Anesthesia
$ 14
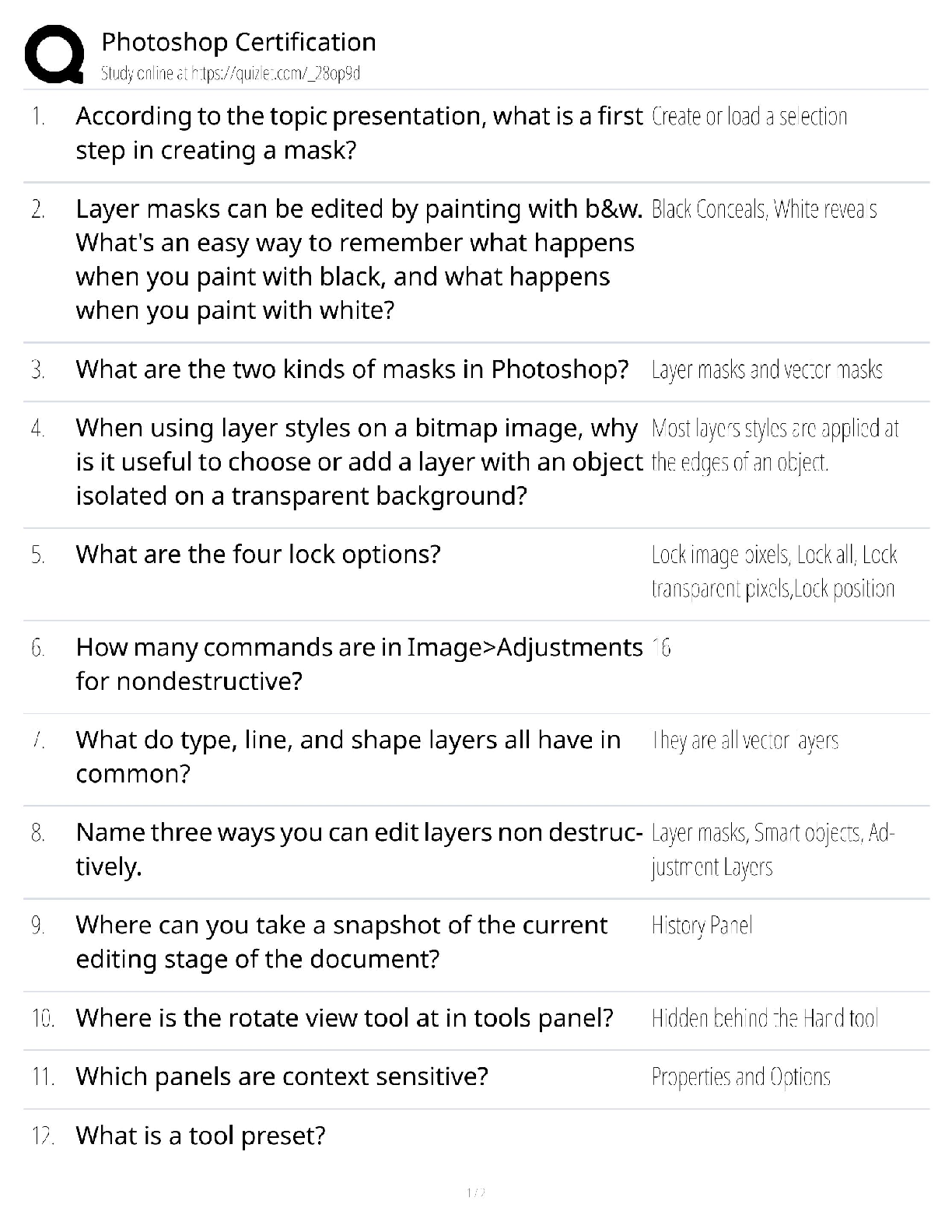
Photoshop Certification / Adobe Certified Professional / 2025 Update / Score 100%
$ 19.5

Instructor's Solution Manual for Horngren's Financial & Managerial Accounting (The Financial Chapters) 8th Edition, Global Edition Miller-Nobles Tracie, Mattison Brenda,
$ 28
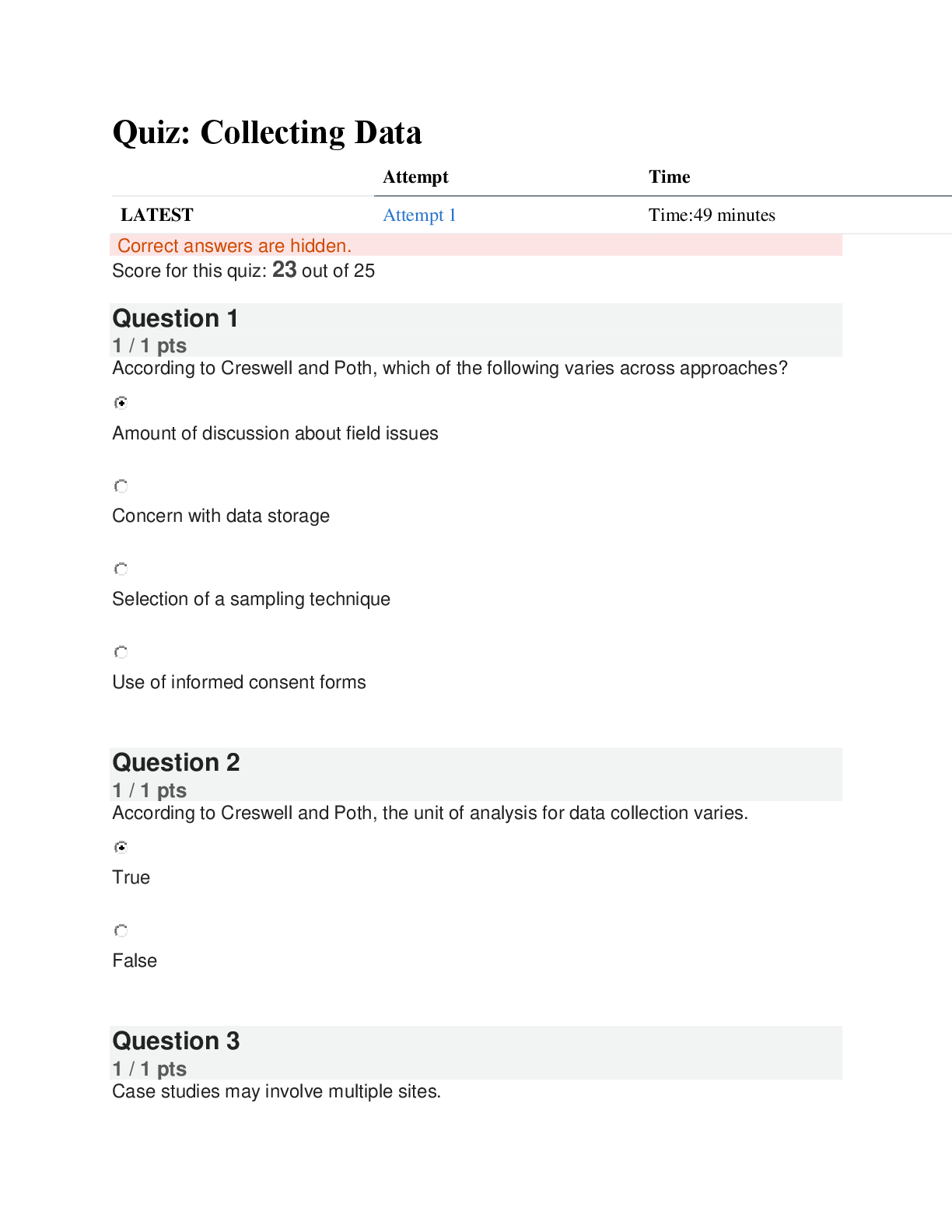
PSYC 810 Quiz: Collecting Data | Score 23/25 | Liberty University
$ 11

Pearson Edexcel_A Level Physical Education_9PE0/02 Question Paper 2020 | Psychological and Social Principles of Physical Education
$ 6.5

RN ATI concept-based assessment, proctored exam for level 4|Test Bank |
$ 15.5

Midterm Quiz 1 with Correct Answers.